Rod visual pigment optimizes active state to achieve efficient G protein activation as compared with cone visual pigments
- PMID: 24375403
- PMCID: PMC3931065
- DOI: 10.1074/jbc.M113.508507
Rod visual pigment optimizes active state to achieve efficient G protein activation as compared with cone visual pigments
Abstract
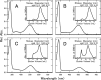
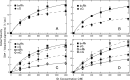
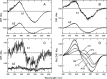
Most vertebrate retinas contain two types of photoreceptor cells, rods and cones, which show different photoresponses to mediate scotopic and photopic vision, respectively. These cells contain different types of visual pigments, rhodopsin and cone visual pigments, respectively, but little is known about the molecular properties of cone visual pigments under physiological conditions, making it difficult to link the molecular properties of rhodopsin and cone visual pigments with the differences in photoresponse between rods and cones. Here we prepared bovine and mouse rhodopsin (bvRh and mRh) and chicken and mouse green-sensitive cone visual pigments (cG and mG) embedded in nanodiscs and applied time-resolved fluorescence spectroscopy to compare their Gt activation efficiencies. Rhodopsin exhibited greater Gt activation efficiencies than cone visual pigments. Especially, the Gt activation efficiency of mRh was about 2.5-fold greater than that of mG at 37 °C, which is consistent with our previous electrophysiological data of knock-in mice. Although the active state (Meta-II) was in equilibrium with inactive states (Meta-I and Meta-III), quantitative determination of Meta-II in the equilibrium showed that the Gt activation efficiency per Meta-II of bvRh was also greater than those of cG and mG. These results indicated that efficient Gt activation by rhodopsin, resulting from an optimized active state of rhodopsin, is one of the causes of the high amplification efficiency of rods.
Keywords: Fluorescence Spectroscopy; G Protein Activation Efficiency; G Protein-coupled Receptors (GPCR); Membrane Bilayer; Membrane Proteins; Rhodopsin; UV Spectroscopy.
Figures






References
-
- Kawamura S., Tachibanaki S. (2008) Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 369–377 - PubMed
-
- Yau K. W. (1994) Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 35, 9–32 - PubMed
-
- Ebrey T., Koutalos Y. (2001) Vertebrate photoreceptors. Prog. Retin. Eye Res. 20, 49–94 - PubMed
-
- Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) G proteins and phototransduction. Annu. Rev. Physiol. 64, 153–187 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

