Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments
- PMID: 24375413
- PMCID: PMC3924264
- DOI: 10.1074/jbc.M113.526350
Spatially restricted G protein-coupled receptor activity via divergent endocytic compartments
Abstract
Postendocytic sorting of G protein-coupled receptors (GPCRs) is driven by their interactions between highly diverse receptor sequence motifs with their interacting proteins, such as postsynaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), zonula occludens-1 protein (zo-1) (PDZ) domain proteins. However, whether these diverse interactions provide an underlying functional specificity, in addition to driving sorting, is unknown. Here we identify GPCRs that recycle via distinct PDZ ligand/PDZ protein pairs that exploit their recycling machinery primarily for targeted endosomal localization and signaling specificity. The luteinizing hormone receptor (LHR) and β2-adrenergic receptor (B2AR), two GPCRs sorted to the regulated recycling pathway, underwent divergent trafficking to distinct endosomal compartments. Unlike B2AR, which traffics to early endosomes (EE), LHR internalizes to distinct pre-early endosomes (pre-EEs) for its recycling. Pre-EE localization required interactions of the LHR C-terminal tail with the PDZ protein GAIP-interacting protein C terminus, inhibiting its traffic to EEs. Rerouting the LHR to EEs, or EE-localized GPCRs to pre-EEs, spatially reprograms MAPK signaling. Furthermore, LHR-mediated activation of MAPK signaling requires internalization and is maintained upon loss of the EE compartment. We propose that combinatorial specificity between GPCR sorting sequences and interacting proteins dictates an unprecedented spatiotemporal control in GPCR signal activity.
Keywords: Endocytosis; Endosomes; G Protein-coupled Receptor (GPCR); GAIP-interacting Protein C Terminus (GIPC); Receptor Recycling; Signaling; Sorting.
Figures


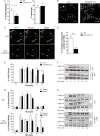
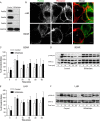

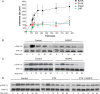
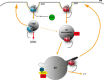
Similar articles
-
Integration of GPCR Signaling and Sorting from Very Early Endosomes via Opposing APPL1 Mechanisms.Cell Rep. 2017 Dec 5;21(10):2855-2867. doi: 10.1016/j.celrep.2017.11.023. Cell Rep. 2017. PMID: 29212031 Free PMC article.
-
The golgi-associated PDZ domain protein PIST/GOPC stabilizes the β1-adrenergic receptor in intracellular compartments after internalization.J Biol Chem. 2015 Mar 6;290(10):6120-9. doi: 10.1074/jbc.M114.605725. Epub 2015 Jan 22. J Biol Chem. 2015. PMID: 25614626 Free PMC article.
-
Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling.J Biol Chem. 2013 May 24;288(21):15023-34. doi: 10.1074/jbc.M113.473660. Epub 2013 Apr 10. J Biol Chem. 2013. PMID: 23576434 Free PMC article.
-
Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity.Adv Pharmacol. 2011;62:279-314. doi: 10.1016/B978-0-12-385952-5.00003-8. Adv Pharmacol. 2011. PMID: 21907913 Free PMC article. Review.
-
Postendocytic Sorting of Adrenergic and Opioid Receptors: New Mechanisms and Functions.Prog Mol Biol Transl Sci. 2015;132:189-206. doi: 10.1016/bs.pmbts.2015.03.005. Epub 2015 Apr 11. Prog Mol Biol Transl Sci. 2015. PMID: 26055059 Free PMC article. Review.
Cited by
-
Recent advances in understanding gonadotropin signaling.Fac Rev. 2021 Apr 19;10:41. doi: 10.12703/r/10-41. eCollection 2021. Fac Rev. 2021. PMID: 34046645 Free PMC article. Review.
-
Identification of novel transplantable GPCR recycling motif for drug discovery.Biochem Pharmacol. 2016 Nov 15;120:22-32. doi: 10.1016/j.bcp.2016.09.011. Epub 2016 Sep 16. Biochem Pharmacol. 2016. PMID: 27645110 Free PMC article.
-
Cellular Heterogeneity of the Luteinizing Hormone Receptor and Its Significance for Cyclic GMP Signaling in Mouse Preovulatory Follicles.Endocrinology. 2020 Jul 1;161(7):bqaa074. doi: 10.1210/endocr/bqaa074. Endocrinology. 2020. PMID: 32384146 Free PMC article.
-
REPLACR-mutagenesis, a one-step method for site-directed mutagenesis by recombineering.Sci Rep. 2016 Jan 11;6:19121. doi: 10.1038/srep19121. Sci Rep. 2016. PMID: 26750263 Free PMC article.
-
Spatiotemporal regulation of the hepatocyte growth factor receptor MET activity by sorting nexins 1/2 in HCT116 colorectal cancer cells.Biosci Rep. 2024 Jun 26;44(6):BSR20240182. doi: 10.1042/BSR20240182. Biosci Rep. 2024. PMID: 38836326 Free PMC article.
References
-
- Stallaert W., Christopoulos A., Bouvier M. (2011) Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert Opin. Drug. Discov. 6, 811–825 - PubMed
-
- Durieux A. C., Prudhon B., Guicheney P., Bitoun M. (2010) Dynamin 2 and human diseases. J. Mol. Med. 88, 339–350 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

