Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection
- PMID: 24375466
- PMCID: PMC3999251
- DOI: 10.1002/hep.26976
Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection
Abstract
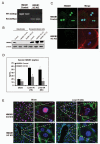
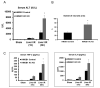
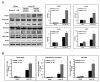
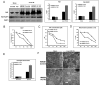
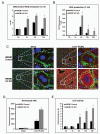
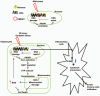
High-mobility group box 1 (HMGB1) is an abundant chromatin-associated nuclear protein and released into the extracellular milieu during liver ischemia-reperfusion (I/R), signaling activation of proinflammatory cascades. Because the intracellular function of HMGB1 during sterile inflammation of I/R is currently unknown, we sought to determine the role of intracellular HMGB1 in hepatocytes after liver I/R. When hepatocyte-specific HMGB1 knockout (HMGB1-HC-KO) and control mice were subjected to a nonlethal warm liver I/R, it was found that HMGB1-HC-KO mice had significantly greater hepatocellular injury after I/R, compared to control mice. Additionally, there was significantly greater DNA damage and decreased chromatin accessibility to repair with lack of HMGB1. Furthermore, lack of hepatocyte HMGB1 led to excessive poly(ADP-ribose)polymerase 1 activation, exhausting nicotinamide adenine dinucleotide and adenosine triphosphate stores, exacerbating mitochondrial instability and damage, and, consequently, leading to increased cell death. We found that this was also associated with significantly more oxidative stress (OS) in HMGB1-HC-KO mice, compared to control. Increased nuclear instability led to a resultant increase in the release of histones with subsequently more inflammatory cytokine production and organ damage through activation of Toll-like receptor 9.
Conclusion: The lack of HMGB1 within hepatocytes leads to increased susceptibility to cellular death after OS conditions.
© 2014 by the American Association for the Study of Liver Diseases.
Figures

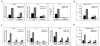
Similar articles
-
Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury.Hepatology. 2010 Feb;51(2):621-32. doi: 10.1002/hep.23365. Hepatology. 2010. PMID: 19902481 Free PMC article.
-
Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling.Mol Med. 2015 Mar 13;20(1):639-48. doi: 10.2119/molmed.2014.00076. Mol Med. 2015. PMID: 25375408 Free PMC article.
-
Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice.Hepatology. 2013 Jul;58(1):374-87. doi: 10.1002/hep.26346. Epub 2013 May 27. Hepatology. 2013. PMID: 23460269 Free PMC article.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
High-mobility group box 1, oxidative stress, and disease.Antioxid Redox Signal. 2011 Apr 1;14(7):1315-35. doi: 10.1089/ars.2010.3356. Antioxid Redox Signal. 2011. PMID: 20969478 Free PMC article. Review.
Cited by
-
Novel Mechanisms of Herbal Therapies for Inhibiting HMGB1 Secretion or Action.Evid Based Complement Alternat Med. 2015;2015:456305. doi: 10.1155/2015/456305. Epub 2015 Mar 2. Evid Based Complement Alternat Med. 2015. PMID: 25821489 Free PMC article. Review.
-
Structure and Functions of HMGB3 Protein.Int J Mol Sci. 2024 Jul 12;25(14):7656. doi: 10.3390/ijms25147656. Int J Mol Sci. 2024. PMID: 39062899 Free PMC article. Review.
-
Redox-dependent regulation of hepatocyte absent in melanoma 2 inflammasome activation in sterile liver injury in mice.Hepatology. 2017 Jan;65(1):253-268. doi: 10.1002/hep.28893. Epub 2016 Nov 29. Hepatology. 2017. PMID: 27774630 Free PMC article.
-
The role of HMGB1/RAGE/TLR4 signaling pathways in cigarette smoke-induced inflammation in chronic obstructive pulmonary disease.Immun Inflamm Dis. 2022 Nov;10(11):e711. doi: 10.1002/iid3.711. Immun Inflamm Dis. 2022. PMID: 36301039 Free PMC article. Review.
-
Targeting HMGB1: A Potential Therapeutic Strategy for Chronic Kidney Disease.Int J Biol Sci. 2023 Sep 25;19(15):5020-5035. doi: 10.7150/ijbs.87964. eCollection 2023. Int J Biol Sci. 2023. PMID: 37781525 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
