Accurate prediction of drug-induced liver injury using stem cell-derived populations
- PMID: 24375539
- PMCID: PMC3925059
- DOI: 10.5966/sctm.2013-0146
Accurate prediction of drug-induced liver injury using stem cell-derived populations
Abstract
Despite major progress in the knowledge and management of human liver injury, there are millions of people suffering from chronic liver disease. Currently, the only cure for end-stage liver disease is orthotopic liver transplantation; however, this approach is severely limited by organ donation. Alternative approaches to restoring liver function have therefore been pursued, including the use of somatic and stem cell populations. Although such approaches are essential in developing scalable treatments, there is also an imperative to develop predictive human systems that more effectively study and/or prevent the onset of liver disease and decompensated organ function. We used a renewable human stem cell resource, from defined genetic backgrounds, and drove them through developmental intermediates to yield highly active, drug-inducible, and predictive human hepatocyte populations. Most importantly, stem cell-derived hepatocytes displayed equivalence to primary adult hepatocytes, following incubation with known hepatotoxins. In summary, we have developed a serum-free, scalable, and shippable cell-based model that faithfully predicts the potential for human liver injury. Such a resource has direct application in human modeling and, in the future, could play an important role in developing renewable cell-based therapies.
Keywords: Cytochrome P450; Drug metabolism; Drug toxicity; Human embryonic stem cells; Liver; iPSC.
Figures


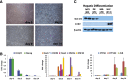

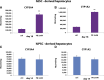
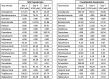
References
-
- British Association for the Study of the Liver. A Time to Act: Improving Liver Health and Outcomes in Liver Disease. Available at http://www.bsg.org.uk/attachments/1004_National%20Liver%20Plan%202009.pdf. Accessed August 1, 2014.
-
- Adam R, Cailliez V, Majno P, et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621–627. - PubMed
-
- Faber W, Seehofer D, Puhl G, et al. Donor age does not influence 12-month outcome after orthotopic liver transplantation. Transplant Proc. 2011;43:3789–3795. - PubMed
-
- Dhawan A, Strom SC, Sokal E, et al. Human hepatocyte transplantation. Methods Mol Biol. 2010;640:525–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

