ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis
- PMID: 24375628
- PMCID: PMC3927960
- DOI: 10.1002/emmm.201303373
ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis
Abstract
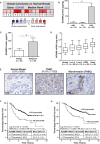
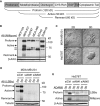
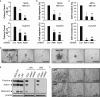
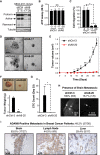
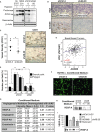
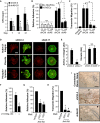
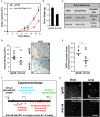
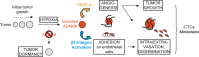
The transmembrane metalloprotease-disintegrin ADAM8 mediates cell adhesion and shedding of ligands, receptors and extracellular matrix components. Here, we report that ADAM8 is abundantly expressed in breast tumors and derived metastases compared to normal tissue, especially in triple-negative breast cancers (TNBCs). Furthermore, high ADAM8 levels predicted poor patient outcome. Consistently, ADAM8 promoted an aggressive phenotype of TNBC cells in culture. In a mouse orthotopic model, tumors derived from TNBC cells with ADAM8 knockdown failed to grow beyond a palpable size and displayed poor vascularization. Circulating tumor cells and brain metastases were also significantly reduced. Mechanistically, ADAM8 stimulated both angiogenesis through release of VEGF-A and transendothelial cell migration via β1-integrin activation. In vivo, treatment with an anti-ADAM8 antibody from the time of cell inoculation reduced primary tumor burden and metastases. Furthermore, antibody treatment of established tumors profoundly decreased metastases in a resection model. As a non-essential protein under physiological conditions, ADAM8 represents a promising novel target for treatment of TNBCs, which currently lack targeted therapies and frequently progress with fatal dissemination.
Figures
Similar articles
-
miR-720 is a downstream target of an ADAM8-induced ERK signaling cascade that promotes the migratory and invasive phenotype of triple-negative breast cancer cells.Breast Cancer Res. 2016 Apr 2;18(1):40. doi: 10.1186/s13058-016-0699-z. Breast Cancer Res. 2016. PMID: 27039296 Free PMC article.
-
ADAM8 expression in breast cancer derived brain metastases: Functional implications on MMP-9 expression and transendothelial migration in breast cancer cells.Int J Cancer. 2018 Feb 15;142(4):779-791. doi: 10.1002/ijc.31090. Epub 2017 Oct 31. Int J Cancer. 2018. PMID: 28986926
-
ADAM8 in invasive cancers: links to tumor progression, metastasis, and chemoresistance.Clin Sci (Lond). 2019 Jan 11;133(1):83-99. doi: 10.1042/CS20180906. Print 2019 Jan 15. Clin Sci (Lond). 2019. PMID: 30635388 Review.
-
ADAM8 as a drug target in pancreatic cancer.Nat Commun. 2015 Jan 28;6:6175. doi: 10.1038/ncomms7175. Nat Commun. 2015. PMID: 25629724 Free PMC article.
-
ADAM8 in allergy.Inflamm Allergy Drug Targets. 2008 Jun;7(2):108-12. doi: 10.2174/187152808785107598. Inflamm Allergy Drug Targets. 2008. PMID: 18691140 Review.
Cited by
-
A Disintegrin and Metalloproteinase (ADAM) Family-Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies?Cancers (Basel). 2022 May 6;14(9):2307. doi: 10.3390/cancers14092307. Cancers (Basel). 2022. PMID: 35565436 Free PMC article. Review.
-
Identification of Genetic and Epigenetic Variants Associated with Breast Cancer Prognosis by Integrative Bioinformatics Analysis.Cancer Inform. 2017 Jan 9;16:1-13. doi: 10.4137/CIN.S39783. eCollection 2017. Cancer Inform. 2017. PMID: 28096648 Free PMC article.
-
Intravenous leiomyomatosis is inclined to a solid entity different from uterine leiomyoma based on RNA-seq analysis with RT-qPCR validation.Cancer Med. 2020 Jul;9(13):4581-4592. doi: 10.1002/cam4.3098. Epub 2020 May 5. Cancer Med. 2020. PMID: 32372565 Free PMC article.
-
Phosphorylation of OGFOD1 by Cell Cycle-Dependent Kinase 7/9 Enhances the Transcriptional Activity of RNA Polymerase II in Breast Cancer Cells.Cancers (Basel). 2021 Jul 8;13(14):3418. doi: 10.3390/cancers13143418. Cancers (Basel). 2021. PMID: 34298635 Free PMC article.
-
EpCAM-Independent Enrichment of Circulating Tumor Cells in Metastatic Breast Cancer.PLoS One. 2015 Dec 22;10(12):e0144535. doi: 10.1371/journal.pone.0144535. eCollection 2015. PLoS One. 2015. PMID: 26695635 Free PMC article.
References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. - PubMed
-
- Bartsch JW, Wildeboer D, Koller G, Naus S, Rittger A, Moss ML, Minai Y, Jockusch H. Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection. J Neurosci. 2010;30:12210–12218. - PMC - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

