Redirecting splicing with bifunctional oligonucleotides
- PMID: 24375754
- PMCID: PMC3973305
- DOI: 10.1093/nar/gkt1287
Redirecting splicing with bifunctional oligonucleotides
Abstract
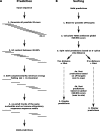
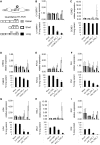
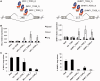
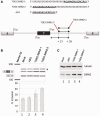
Ectopic modulators of alternative splicing are important tools to study the function of splice variants and for correcting mis-splicing events that cause human diseases. Such modulators can be bifunctional oligonucleotides made of an antisense portion that determines target specificity, and a non-hybridizing tail that recruits proteins or RNA/protein complexes that affect splice site selection (TOSS and TOES, respectively, for targeted oligonucleotide silencer of splicing and targeted oligonucleotide enhancer of splicing). The use of TOSS and TOES has been restricted to a handful of targets. To generalize the applicability and demonstrate the robustness of TOSS, we have tested this approach on more than 50 alternative splicing events. Moreover, we have developed an algorithm that can design active TOSS with a success rate of 80%. To produce bifunctional oligonucleotides capable of stimulating splicing, we built on the observation that binding sites for TDP-43 can stimulate splicing and improve U1 snRNP binding when inserted downstream from 5' splice sites. A TOES designed to recruit TDP-43 improved exon 7 inclusion in SMN2. Overall, our study shows that bifunctional oligonucleotides can redirect splicing on a variety of genes, justifying their inclusion in the molecular arsenal that aims to alter the production of splice variants.
Figures




References
-
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008;40:1413–1415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

