Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs
- PMID: 24375796
- PMCID: PMC3920226
- DOI: 10.1152/ajplung.00264.2013
Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs
Abstract
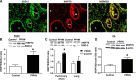
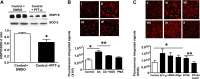
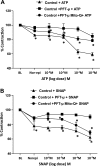
Superoxide dismutase 2 (SOD-2) is synthesized in the cytosol and imported into the mitochondrial matrix, where it is activated and functions as the primary antioxidant for cellular respiration. The specific mechanisms that target SOD-2 to the mitochondria remain unclear. We hypothesize that inducible heat shock protein 70 (iHSP70) targets SOD-2 to the mitochondria via a mechanism facilitated by ATP, and this process is impaired in persistent pulmonary hypertension of the newborn (PPHN). We observed that iHSP70 interacts with SOD-2 and targets SOD-2 to the mitochondria. Interruption of iHSP70-SOD-2 interaction with 2-phenylethylenesulfonamide-μ (PFT-μ, a specific inhibitor of substrate binding to iHSP70 COOH terminus) and siRNA-mediated knockdown of iHSP70 expression disrupted SOD-2 transport to mitochondria. Increasing intracellular ATP levels by stimulation of respiration with CaCl2 facilitated the mitochondrial import of SOD-2, increased SOD-2 activity, and decreased the mitochondrial superoxide (O2(·-)) levels in PPHN pulmonary artery endothelial cells (PAEC) by promoting iHSP70-SOD-2 dissociation at the outer mitochondrial membrane. In contrast, oligomycin, an inhibitor of mitochondrial ATPase, decreased SOD-2 expression and activity and increased O2(·-) levels in the mitochondria of control PAEC. The basal ATP levels and degree of iHSP70-SOD-2 dissociation were lower in PPHN PAEC and lead to increased SOD-2 degradation in cytosol. In normal pulmonary arteries (PA), PFT-μ impaired the relaxation response of PA rings in response to nitric oxide (NO) donor, S-nitroso-N-acetyl-penicillamine. Pretreatment with Mito-Q, a mitochondrial targeted O2(·-) scavenger, restored the relaxation response in PA rings pretreated with PFT-μ. Our observations suggest that iHSP70 chaperones SOD-2 to the mitochondria. Impaired SOD-2-iHSP70 dissociation decreases SOD-2 import and contributes to mitochondrial oxidative stress in PPHN.
Keywords: nitric oxide; oxidative stress; persistent pulmonary hypertension of the newborn; pulmonary artery endothelium; vasodilation.
Figures

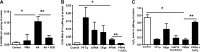
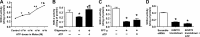
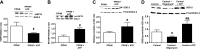

References
-
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990 - PubMed
-
- Azem A, Oppliger W, Lustig A, Jeno ÈP, Feifel B, Schatz G, Horst M. The mitochondrial hsp70 chaperone system, Effect of adenine nucleotides, peptide substrate and mGrpE on the oligomeric state of mhsp70. J Biol Chem 272: 20901–20906, 1997 - PubMed
-
- Beddoe T, Lithgow T. Delivery of nascent polypeptides to the mitochondrial surface. Biochim Biophys Acta 1592: 35–39, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

