A general method for artificial metalloenzyme formation through strain-promoted azide-alkyne cycloaddition
- PMID: 24376040
- PMCID: PMC3996923
- DOI: 10.1002/cbic.201300661
A general method for artificial metalloenzyme formation through strain-promoted azide-alkyne cycloaddition
Abstract
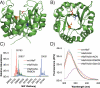
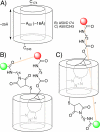
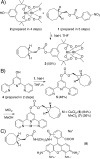
Strain-promoted azide-alkyne cycloaddition (SPAAC) can be used to generate artificial metalloenzymes (ArMs) from scaffold proteins containing a p-azido-L-phenylalanine (Az) residue and catalytically active bicyclononyne-substituted metal complexes. The high efficiency of this reaction allows rapid ArM formation when using Az residues within the scaffold protein in the presence of cysteine residues or various reactive components of cellular lysate. In general, cofactor-based ArM formation allows the use of any desired metal complex to build unique inorganic protein materials. SPAAC covalent linkage further decouples the native function of the scaffold from the installation process because it is not affected by native amino acid residues; as long as an Az residue can be incorporated, an ArM can be generated. We have demonstrated the scope of this method with respect to both the scaffold and cofactor components and established that the dirhodium ArMs generated can catalyze the decomposition of diazo compounds and both Si-H and olefin insertion reactions involving these carbene precursors.
Keywords: artificial metalloenzymes; biocatalysis; click chemistry; cofactors; dirhodium.
Copyright © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Lewis JC. ACS Catal. Just Accepted Manuscript, DOI: 10.1021/cs400806a.
- Rosati F, Roelfes G. ChemCatChem. 2010;2:916–927.
- Heinisch T, Ward TR. Curr. Opin. Chem. Biol. 2010;14:184–199. - PubMed
- Lu Y, Yeung N, Sieracki N, Marshall NM. Nature. 2009;460:855–862. - PMC - PubMed
- Abe S, Ueno T, Watanabe Y. Top. Organomet. Chem. 2009;25:25–43.
-
- Wilson ME, Whitesides GM. J. Am. Chem. Soc. 1978;100:306–307.
- Mao J, Ward TR. Chimia. 2008;62:956–961.
- Allard M, Dupont C, Robles VM, Doucet N, Lledos A, Maréchal JD, Urvoas A, Mahy JP, Ricoux R. ChemBioChem. 2011;13:240–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

