Aqueous oxidative Heck reaction as a protein-labeling strategy
- PMID: 24376051
- PMCID: PMC4159585
- DOI: 10.1002/cbic.201300714
Aqueous oxidative Heck reaction as a protein-labeling strategy
Abstract
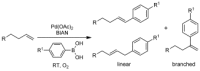
An increasing number of chemical reactions are being employed for bio-orthogonal ligation of detection labels to protein-bound functional groups. Several of these strategies, however, are limited in their application to pure proteins and are ineffective in complex biological samples such as cell lysates. Here we present the palladium-catalyzed oxidative Heck reaction as a new and robust bio-orthogonal strategy for linking functionalized arylboronic acids to protein-bound alkenes in high yields and with excellent chemoselectivity even in the presence of complex protein mixtures from living cells. Advantageously, this reaction proceeds under aerobic conditions, whereas most other metal-catalyzed reactions require inert atmosphere.
Keywords: Heck reaction; cross-coupling; homogeneous catalysis; palladium; protein modifications.
© 2013 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Figures
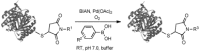

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

