Mathematical Models of the Impact of IL2 Modulation Therapies on T Cell Dynamics
- PMID: 24376444
- PMCID: PMC3858650
- DOI: 10.3389/fimmu.2013.00439
Mathematical Models of the Impact of IL2 Modulation Therapies on T Cell Dynamics
Abstract
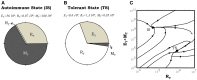
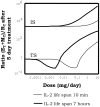
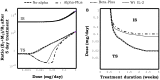
Several reports in the literature have drawn a complex picture of the effect of treatments aiming to modulate IL2 activity in vivo. They seem to promote either immunity or tolerance, probably depending on the specific context, dose, and timing of their application. Such complexity might derive from the pleiotropic role of IL2 in T cell dynamics. To theoretically address the latter possibility, our group has developed several mathematical models for Helper, Regulatory, and Memory T cell population dynamics, which account for most well-known facts concerning their relationship with IL2. We have simulated the effect of several types of therapies, including the injection of: IL2; antibodies anti-IL2; IL2/anti-IL2 immune-complexes; and mutant variants of IL2. We studied the qualitative and quantitative conditions of dose and timing for these treatments which allow them to potentiate either immunity or tolerance. Our results provide reasonable explanations for the existent pre-clinical and clinical data, predict some novel treatments, and further provide interesting practical guidelines to optimize the future application of these types of treatments.
Keywords: T cell dynamics; interleukin 2; interleukin 2 mutants; mathematical model; regulatory T cells.
Figures







References
-
- Fishman M, Hunter TB, Soliman H, Thompson P, Dunn M, Smilee R, et al. Phase II trial of B7-1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J Immunother (2008) 31(1):72–80 10.1097/CJI.0b013e31815ba792 - DOI - PubMed
-
- Tarpey I, van Loon AA, de Haas N, Davis PJ, Orbell S, Cavanagh D, et al. A recombinant turkey Herpesvirus expressing chicken interleukin-2 increases the protection provided by in ovo vaccination with infectious bursal disease and infectious bronchitis virus. Vaccine (2007) 25(51):8529–35 10.1016/j.vaccine.2007.10.006 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

