IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis
- PMID: 24376495
- PMCID: PMC3869650
- DOI: 10.1371/journal.pone.0080288
IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis
Erratum in
- PLoS One. 2014;9(3):e91197
Abstract
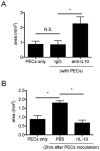
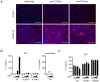
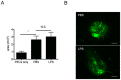
Subretinal fibrosis is directly related to severe visual loss, especially if occurs in the macula, and is frequently observed in advanced age-related macular degeneration and other refractory eye disorders such as diabetic retinopathy and uveitis. In this study, we analyzed the immunosuppressive mechanism of subretinal fibrosis using the novel animal model recently demonstrated. Both TLR2 and TLR4 deficient mice showed significant enlargement of subretinal fibrotic area as compared with wild-type mice. A single intraocular administration of heat shock protein 70 (HSP70), which is an endogenous ligand for TLR2 and TLR4, inhibited subretinal fibrosis in wild-type mice but not in TLR2 and TLR4-deficient mice. Additionally, HSP70 induced IL-10 production in eyes from wild-type mice but was impaired in both TLR2- and TLR4-deficient mice, indicating that HSP70-TLR2/TLR4 axis plays an immunomodulatory role in subretinal fibrosis. Thus, these results suggest that HSP70-TLR2/TLR4 axis is a new therapeutic target for subretinal fibrosis due to prognostic CNV.
Conflict of interest statement
Figures




References
-
- Bressler NM (2004) Age-related macular degeneration is the leading cause of blindness… JAMA. 291: 1900–1901. - PubMed
-
- Miller JW (2011) Treatment of age-related macular degeneration: Beyond VEGF. Jpn J Ophthalmol 54: 523–528. - PubMed
-
- Stramer BM, Mori R, Martin P (2007) The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol 127: 1009–1017. - PubMed
-
- van Eden W, van der Zee R, Prakken B (2005) Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5: 318–330. - PubMed
-
- Ludwig D, Stahl M, Ibrahim ET, Wenzel BE, Drabicki D, et al. (1999) Enhanced intestinal expression of heat shock protein 70 in patients with inflammatory bowel diseases. Dig Dis Sci 44: 1440–1447. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

