Nitric oxide in plants: the roles of ascorbate and hemoglobin
- PMID: 24376554
- PMCID: PMC3869716
- DOI: 10.1371/journal.pone.0082611
Nitric oxide in plants: the roles of ascorbate and hemoglobin
Abstract
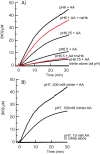
Ascorbic acid and hemoglobins have been linked to nitric oxide metabolism in plants. It has been hypothesized that ascorbic acid directly reduces plant hemoglobin in support of NO scavenging, producing nitrate and monodehydroascorbate. In this scenario, monodehydroascorbate reductase uses NADH to reduce monodehydroascorbate back to ascorbate to sustain the cycle. To test this hypothesis, rates of rice nonsymbiotic hemoglobin reduction by ascorbate were measured directly, in the presence and absence of purified rice monodehydroascorbate reductase and NADH. Solution NO scavenging was also measured methodically in the presence and absence of rice nonsymbiotic hemoglobin and monodehydroascorbate reductase, under hypoxic and normoxic conditions, in an effort to gauge the likelihood of these proteins affecting NO metabolism in plant tissues. Our results indicate that ascorbic acid slowly reduces rice nonsymbiotic hemoglobin at a rate identical to myoglobin reduction. The product of the reaction is monodehydroascorbate, which can be efficiently reduced back to ascorbate in the presence of monodehydroascorbate reductase and NADH. However, our NO scavenging results suggest that the direct reduction of plant hemoglobin by ascorbic acid is unlikely to serve as a significant factor in NO metabolism, even in the presence of monodehydroascorbate reductase. Finally, the possibility that the direct reaction of nitrite/nitrous acid and ascorbic acid produces NO was measured at various pH values mimicking hypoxic plant cells. Our results suggest that this reaction is a likely source of NO as the plant cell pH drops below 7, and as nitrite concentrations rise to mM levels during hypoxia.
Conflict of interest statement
Figures





References
-
- Smirnoff N (1996) The Function and Metabolism of Ascorbic Acid in Plants. Annals of botany 78: 661–669.
-
- Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35: 291–314. - PubMed
-
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3: 229–235. - PubMed
-
- Foyer CH (1993) Ascorbic acid. In: Alscher RG, Hess JL, editors. Antioxidants in higher plants: CRC Press. 31–58.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

