Cyclic AMP-dependent resuscitation of dormant Mycobacteria by exogenous free fatty acids
- PMID: 24376605
- PMCID: PMC3871856
- DOI: 10.1371/journal.pone.0082914
Cyclic AMP-dependent resuscitation of dormant Mycobacteria by exogenous free fatty acids
Erratum in
- PLoS One. 2014;9(5):e97206
Abstract
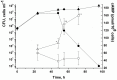
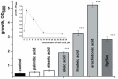
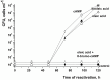
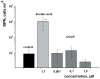
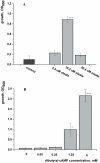
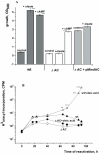
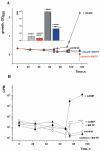
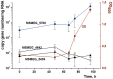
One third of the world population carries a latent tuberculosis (TB) infection, which may reactivate leading to active disease. Although TB latency has been known for many years it remains poorly understood. In particular, substances of host origin, which may induce the resuscitation of dormant mycobacteria, have not yet been described. In vitro models of dormant ("non-culturable") cells of Mycobacterium smegmatis (mc(2)155) and Mycobacterium tuberculosis H37Rv were used. We found that the resuscitation of dormant M. smegmatis and M. tuberculosis cells in liquid medium was stimulated by adding free unsaturated fatty acids (FA), including arachidonic acid, at concentrations of 1.6-10 µM. FA addition enhanced cAMP levels in reactivating M. smegmatis cells and exogenously added cAMP (3-10 mM) or dibutyryl-cAMP (0.5-1 mM) substituted for FA, causing resuscitation of M. smegmatis and M. tuberculosis dormant cells. A M. smegmatis null-mutant lacking MSMEG_4279, which encodes a FA-activated adenylyl cyclase (AC), could not be resuscitated by FA but it was resuscitated by cAMP. M. smegmatis and M. tuberculosis cells hyper-expressing AC were unable to form non-culturable cells and a specific inhibitor of AC (8-bromo-cAMP) prevented FA-dependent resuscitation. RT-PCR analysis revealed that rpfA (coding for resuscitation promoting factor A) is up-regulated in M. smegmatis in the beginning of exponential growth following the cAMP increase in lag phase caused by FA-induced cell activation. A specific Rpf inhibitor (4-benzoyl-2-nitrophenylthiocyanate) suppressed FA-induced resuscitation. We propose a novel pathway for the resuscitation of dormant mycobacteria involving the activation of adenylyl cyclase MSMEG_4279 by FAs resulted in activation of cellular metabolism followed later by increase of RpfA activity which stimulates cell multiplication in exponential phase. The study reveals a probable role for lipids of host origin in the resuscitation of dormant mycobacteria, which may function during the reactivation of latent TB.
Conflict of interest statement
Figures

References
-
- Lillebaek T, Kok-Jensen A, Viskum K (2002) Bacillarity at autopsy in pulmonary tuberculosis. Mycobacterium tuberculosis is often disseminated. Apmis 110(9): 625–9. - PubMed
-
- Chao MC, Rubin EJ (2010) Letting sleeping dos Lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64: 293–311. - PubMed
-
- Shleeva MO, Bagramyan K, Telkov MV, Mukamolova GV, Young M, et al. (2002) Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology 148(5): 1581–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

