Rotor termination is critically dependent on kinetic properties of I kur inhibitors in an in silico model of chronic atrial fibrillation
- PMID: 24376659
- PMCID: PMC3869770
- DOI: 10.1371/journal.pone.0083179
Rotor termination is critically dependent on kinetic properties of I kur inhibitors in an in silico model of chronic atrial fibrillation
Abstract
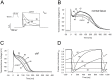
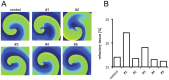
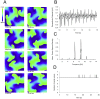
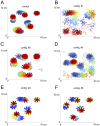
Inhibition of the atrial ultra-rapid delayed rectifier potassium current (I Kur) represents a promising therapeutic strategy in the therapy of atrial fibrillation. However, experimental and clinical data on the antiarrhythmic efficacy remain controversial. We tested the hypothesis that antiarrhythmic effects of I Kur inhibitors are dependent on kinetic properties of channel blockade. A mathematical description of I Kur blockade was introduced into Courtemanche-Ramirez-Nattel models of normal and remodeled atrial electrophysiology. Effects of five model compounds with different kinetic properties were analyzed. Although a reduction of dominant frequencies could be observed in two dimensional tissue simulations for all compounds, a reduction of spiral wave activity could be only be detected in two cases. We found that an increase of the percent area of refractory tissue due to a prolongation of the wavelength seems to be particularly important. By automatic tracking of spiral tip movement we find that increased refractoriness resulted in rotor extinction caused by an increased spiral-tip meandering. We show that antiarrhythmic effects of I Kur inhibitors are dependent on kinetic properties of blockade. We find that an increase of the percent area of refractory tissue is the underlying mechanism for an increased spiral-tip meandering, resulting in the extinction of re-entrant circuits.
Conflict of interest statement
Figures



References
-
- Nattel S, Yue L, Wang Z (1999) Cardiac ultrarapid delayed rectifiers: a novel potassium current family o f functional similarity and molecular diversity. Cell Physiol Biochem 9: 217–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

