In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis
- PMID: 24376670
- PMCID: PMC3871555
- DOI: 10.1371/journal.pone.0083247
In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis
Abstract
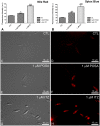
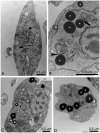
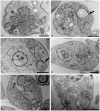
Leishmaniasis, caused by protozoan parasites of the Leishmania genus, is one of the most prevalent neglected tropical diseases. It is endemic in 98 countries, causing considerable morbidity and mortality. Pentavalent antimonials are the first line of treatment for leishmaniasis except in India. In resistant cases, miltefosine, amphotericin B and pentamidine are used. These treatments are unsatisfactory due to toxicity, limited efficacy, high cost and difficult administration. Thus, there is an urgent need to develop drugs that are efficacious, safe, and more accessible to patients. Trypanosomatids, including Leishmania spp. and Trypanosoma cruzi, have an essential requirement for ergosterol and other 24-alkyl sterols, which are absent in mammalian cells. Inhibition of ergosterol biosynthesis is increasingly recognized as a promising target for the development of new chemotherapeutic agents. The aim of this work was to investigate the antiproliferative, physiological and ultrastructural effects against Leishmania amazonensis of itraconazole (ITZ) and posaconazole (POSA), two azole antifungal agents that inhibit sterol C14α-demethylase (CYP51). Antiproliferative studies demonstrated potent activity of POSA and ITZ: for promastigotes, the IC50 values were 2.74 µM and 0.44 µM for POSA and ITZ, respectively, and for intracellular amastigotes, the corresponding values were 1.63 µM and 0.08 µM, for both stages after 72 h of treatment. Physiological studies revealed that both inhibitors induced a collapse of the mitochondrial membrane potential (ΔΨm), which was consistent with ultrastructural alterations in the mitochondrion. Intense mitochondrial swelling, disorganization and rupture of mitochondrial membranes were observed by transmission electron microscopy. In addition, accumulation of lipid bodies, appearance of autophagosome-like structures and alterations in the kinetoplast were also observed. In conclusion, our results indicate that ITZ and POSA are potent inhibitors of L. amazonensis and suggest that these drugs could represent novel therapies for the treatment of leishmaniasis, either alone or in combination with other agents.
Conflict of interest statement
Figures






References
-
- Herwaldt BL (1999) Leishmaniasis. Lancet 354: 1191–1199. - PubMed
-
- Grimaldi G Jr, McMahan-Pratt D (1991) Leishmaniasis and its etiologic agents in the New World: an overview. Prog Clin Parasitol 2: 73–118. - PubMed
-
- Liew FY, O’Donnell CA (1993) Immunology of leishmaniasis. Adv Parasitol 32: 161–259. - PubMed
-
- Barral A, Pedral-Sampaio D, Grimaldi Jr G, Momen H, McMahon-Pratt D, et al. (1991) Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg 44: 536–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

