At a supra-physiological concentration, human sexual hormones act as quorum-sensing inhibitors
- PMID: 24376718
- PMCID: PMC3871529
- DOI: 10.1371/journal.pone.0083564
At a supra-physiological concentration, human sexual hormones act as quorum-sensing inhibitors
Abstract
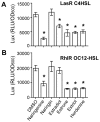
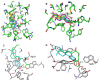
N-acylhomoserine lactone (AHL)-mediated quorum-sensing (QS) regulates virulence functions in plant and animal pathogens such as Agrobacterium tumefaciens and Pseudomonas aeruginosa. A chemolibrary of more than 3500 compounds was screened using two bacterial AHL-biosensors to identify QS-inhibitors (QSIs). The purity and structure of 15 QSIs selected through this screening were verified using HPLC MS/MS tools and their activity tested on the A. tumefaciens and P. aeruginosa bacterial models. The IC50 value of the identified QSIs ranged from 2.5 to 90 µg/ml, values that are in the same range as those reported for the previously identified QSI 4-nitropyridine-N-oxide (IC50 24 µg/ml). Under the tested culture conditions, most of the identified QSIs did not exhibit bacteriostatic or bactericidal activities. One third of the tested QSIs, including the plant compound hordenine and the human sexual hormone estrone, decreased the frequency of the QS-regulated horizontal transfer of the tumor-inducing (Ti) plasmid in A. tumefaciens. Hordenine, estrone as well as its structural relatives estriol and estradiol, also decreased AHL accumulation and the expression of six QS-regulated genes (lasI, lasR, lasB, rhlI, rhlR, and rhlA) in cultures of the opportunist pathogen P. aeruginosa. Moreover, the ectopic expression of the AHL-receptors RhlR and LasR of P. aeruginosa in E. coli showed that their gene-regulatory activity was affected by the QSIs. Finally, modeling of the structural interactions between the human hormones and AHL-receptors LasR of P. aeruginosa and TraR of A. tumefaciens confirmed the competitive binding capability of the human sexual hormones. This work indicates potential interferences between bacterial and eukaryotic hormonal communications.
Conflict of interest statement
Figures
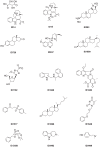

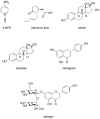
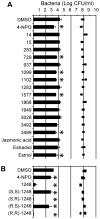

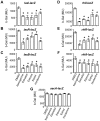
References
-
- Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR (2011) Applications of small molecule activators and inhibitors of quorum-sensing in gram-negative bacteria. Chem Rev 111: 28–67. - PubMed
-
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25: 365–404. - PubMed
-
- Janssens JC, De Keersmaecker SC, De Vos DE, Vanderleyden J (2008) Small molecules for interference with cell-cell-communication systems in Gram-negative bacteria. Curr Med Chem 15: 2144–2156. - PubMed
-
- Amara N, Krom BP, Kaufmann GF, Meijler MM (2011) Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem Rev 111: 195–208. - PubMed
-
- Kalia VC (2013) Quorum sensing inhibitors: An overview. Biotechnol Adv 31: 224–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

