Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation
- PMID: 24376802
- PMCID: PMC3869846
- DOI: 10.1371/journal.pone.0084324
Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation
Abstract
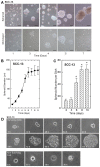
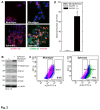
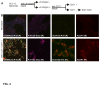
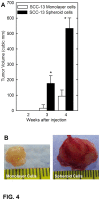
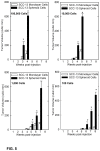
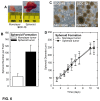
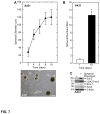
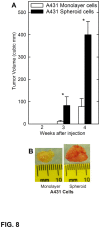
Epidermal squamous cell carcinoma is among the most common cancers in humans. These tumors are comprised of phenotypically diverse populations of cells that display varying potential for proliferation and differentiation. An important goal is identifying cells from this population that drive tumor formation. To enrich for tumor-forming cells, cancer cells were grown as spheroids in non-attached conditions. We show that spheroid-selected cells form faster growing and larger tumors in immune-compromised mice as compared to non-selected cells. Moreover, spheroid-selected cells gave rise to tumors following injection of as few as one hundred cells, suggesting these cells have enhanced tumor-forming potential. Cells isolated from spheroid-selected tumors retain an enhanced ability to grow as spheroids when grown in non-attached culture conditions. Thus, these tumor-forming cells retain their phenotype following in vivo passage as tumors. Detailed analysis reveals that spheroid-selected cultures are highly enriched for expression of epidermal stem cell and embryonic stem cell markers, including aldehyde dehydrogenase 1, keratin 15, CD200, keratin 19, Oct4, Bmi-1, Ezh2 and trimethylated histone H3. These studies indicate that a subpopulation of cells that possess stem cell-like properties and express stem cell markers can be derived from human epidermal cancer cells and that these cells display enhanced ability to drive tumor formation.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

