Defining the role of fluid shear stress in the expression of early signaling markers for calcific aortic valve disease
- PMID: 24376809
- PMCID: PMC3871675
- DOI: 10.1371/journal.pone.0084433
Defining the role of fluid shear stress in the expression of early signaling markers for calcific aortic valve disease
Abstract
Calcific aortic valve disease (CAVD) is an active process presumably triggered by interplays between cardiovascular risk factors, molecular signaling networks and hemodynamic cues. While earlier studies demonstrated that alterations in fluid shear stress (FSS) on the fibrosa could trigger inflammation, the mechanisms of CAVD pathogenesis secondary to side-specific FSS abnormalities are poorly understood. This knowledge could be critical to the elucidation of key CAVD risk factors such as congenital valve defects, aging and hypertension, which are known to generate FSS disturbances. The objective of this study was to characterize ex vivo the contribution of isolated and combined abnormalities in FSS magnitude and frequency to early valvular pathogenesis. The ventricularis and fibrosa of porcine aortic valve leaflets were exposed simultaneously to different combinations of sub-physiologic/physiologic/supra-physiologic levels of FSS magnitude and frequency for 24, 48 and 72 hours in a double cone-and-plate device. Endothelial activation and paracrine signaling were investigated by measuring cell-adhesion molecule (ICAM-1, VCAM-1) and cytokine (BMP-4, TGF-β1) expressions, respectively. Extracellular matrix (ECM) degradation was characterized by measuring the expression and activity of the proteases MMP-2, MMP-9, cathepsin L and cathepsin S. The effect of the FSS treatment yielding the most significant pathological response was examined over a 72-hour period to characterize the time-dependence of FSS mechano-transduction. While cytokine expression was stimulated under elevated FSS magnitude at normal frequency, ECM degradation was stimulated under both elevated FSS magnitude at normal frequency and physiologic FSS magnitude at abnormal frequency. In contrast, combined FSS magnitude and frequency abnormalities essentially maintained valvular homeostasis. The pathological response under supra-physiologic FSS magnitude peaked at 48 hours but was then maintained until the 72-hour time point. This study confirms the sensitivity of valve leaflets to both FSS magnitude and frequency and suggests the ability of supra-physiologic FSS levels or abnormal FSS frequencies to initiate CAVD mechanisms.
Conflict of interest statement
Figures
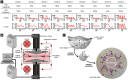



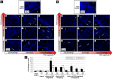
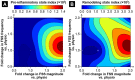
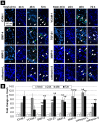

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

