The effects of industry sponsorship on comparator selection in trial registrations for neuropsychiatric conditions in children
- PMID: 24376857
- PMCID: PMC3871546
- DOI: 10.1371/journal.pone.0084951
The effects of industry sponsorship on comparator selection in trial registrations for neuropsychiatric conditions in children
Abstract
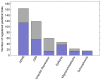
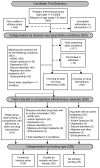
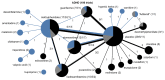
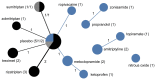
Pediatric populations continue to be understudied in clinical drug trials despite the increasing use of pharmacotherapy in children, particularly with psychotropic drugs. Most pertinent to the clinical selection of drug interventions are trials directly comparing drugs against other drugs. The aim was to measure the prevalence of active drug comparators in neuropsychiatric drug trials in children and identify the effects of funding source on comparator selection. We analyzed the selection of drugs and drug comparisons in clinical trials registered between January 2006 and May 2012. Completed and ongoing interventional trials examining treatments for six neuropsychiatric conditions in children were included. Networks of drug comparisons for each condition were constructed using information about the trial study arms. Of 421 eligible trial registrations, 228 (63,699 participants) were drug trials addressing ADHD (106 trials), autism spectrum disorders (47), unipolar depression (16), seizure disorders (38), migraines and other headaches (15), or schizophrenia (11). Active drug comparators were used in only 11.0% of drug trials while 44.7% used a placebo control and 44.3% no drug or placebo comparator. Even among conditions with well-established pharmacotherapeutic options, almost all drug interventions were compared to a placebo. Active comparisons were more common among trials without industry funding (17% vs. 8%, p=0.04). Trials with industry funding differed from non-industry trials in terms of the drugs studied and the comparators selected. For 73% (61/84) of drugs and 90% (19/21) of unique comparisons, trials were funded exclusively by either industry or non-industry. We found that industry and non-industry differed when choosing comparators and active drug comparators were rare for both groups. This gap in pediatric research activity limits the evidence available to clinicians treating children and suggests a need to reassess the design and funding of pediatric trials in order to optimize the information derived from pediatric participation in clinical trials.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

