IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway
- PMID: 24376862
- PMCID: PMC3871633
- DOI: 10.1371/journal.pone.0085032
IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway
Abstract
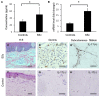
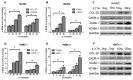
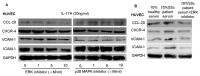
Recent reports have demonstrated that endothelial cells are involved in vascular inflammatory injury in systemic sclerosis (SSc) and interleukin-17A (IL-17A) plays a crucial role in the pathogenesis of SSC. However, little is known about the effects of IL-17A on endothelial cell inflammation in SSC. The aim of our study was to investigate the role of IL-17A in endothelial inflammation. Here, we showed that IL-17A mRNA and protein levels were augmented in the peripheral blood and more IL-17⁺ lymphocytes infiltrated in the perivascular areas in the involved skin of SSC patients. SSC patient serum induced chemokine and adhesion molecule expression in HUVECs, which was blocked by IL-17A neutralization. IL-17A alone induced chemokine and adhesion molecule expression and promoted T cell-HUVEC adhesion. Extracellular signal-regulated kinase (ERK) inhibition and IL-17A neutralization prominently inhibited chemokine and adhesion molecule expression and blocked T cell-HUVEC adhesion. IL-17A derived from SSC patient serum mediated endothelial cells inflammation by up-regulating chemokines and adhesion molecules, which was blocked by ERK inhibition. These data imply that ERK signal pathway might play a key role in the progression of endothelial injury induced by IL-17A in SSC.
Conflict of interest statement
Figures



References
-
- Varga J (2012) Etiology and Pathogenesis of Scleroderma. 9 # ed. pp. 1343-1353
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

