Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization
- PMID: 24377302
- PMCID: PMC3906892
- DOI: 10.1021/nn405701q
Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization
Abstract
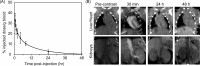
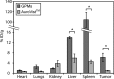
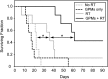
Gold nanoparticles (AuNPs) have generated interest as both imaging and therapeutic agents. AuNPs are attractive for imaging applications since they are nontoxic and provide nearly three times greater X-ray attenuation per unit weight than iodine. As therapeutic agents, AuNPs can sensitize tumor cells to ionizing radiation. To create a nanoplatform that could simultaneously exhibit long circulation times, achieve appreciable tumor accumulation, generate computed tomography (CT) image contrast, and serve as a radiosensitizer, gold-loaded polymeric micelles (GPMs) were prepared. Specifically, 1.9 nm AuNPs were encapsulated within the hydrophobic core of micelles formed with the amphiphilic diblock copolymer poly(ethylene glycol)-b-poly(ε-capralactone). GPMs were produced with low polydispersity and mean hydrodynamic diameters ranging from 25 to 150 nm. Following intravenous injection, GPMs provided blood pool contrast for up to 24 h and improved the delineation of tumor margins via CT. Thus, GPM-enhanced CT imaging was used to guide radiation therapy delivered via a small animal radiation research platform. In combination with the radiosensitizing capabilities of gold, tumor-bearing mice exhibited a 1.7-fold improvement in the median survival time, compared with mice receiving radiation alone. It is envisioned that translation of these capabilities to human cancer patients could guide and enhance the efficacy of radiation therapy.
Figures




References
-
- Benita S.; Poly P. A.; Puisieux F.; Delattre J. Radiopaque Liposomes: Effect of Formulation Conditions on Encapsulation Efficiency. J. Pharm. Sci. 1984, 73, 1751–1755. - PubMed
-
- Elrod D. B.; Partha R.; Danila D.; Casscells S. W.; Conyers J. L. An Iodinated Liposomal Computed Tomographic Contrast Agent Prepared from a Diiodophosphatidylcholine Lipid. Nanomedicine 2009, 5, 42–45. - PubMed
-
- Sachse A.Iodinated Liposomes as Contrast Agents. In Fundamental Biomedical Technologies: Nanoparticles in Biomedical Imaging; Ferrari M., Bulte J. W. M., Modo M. M. J., Eds.; Springer: New York, 2008; pp 371–410.
-
- Torchilin V. P.; Frank-Kamenetsky M. D.; Wolf G. L. CT Visualization of Blood Pool in Rats by Using Long-Circulating, Iodine-Containing Micelles. Acad. Radiol. 1999, 6, 61–65. - PubMed
-
- Long D. M. Jr.; Lasser E. C.; Sharts C. M.; Multer F. K.; Nielsen M. Experiments with Radiopaque Perfluorocarbon Emulsions for Selective Opacification of Organs and Total Body Angiography. Invest. Radiol. 1980, 15, 242–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

