Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast
- PMID: 24378231
- PMCID: PMC3884728
- DOI: 10.3201/eid2001.130906
Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast
Abstract
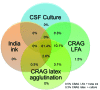
Cryptococcal meningitis is common in sub-Saharan Africa. Given the need for data for a rapid, point-of-care cryptococcal antigen (CRAG) lateral flow immunochromatographic assay (LFA), we assessed diagnostic performance of cerebrospinal fluid (CSF) culture, CRAG latex agglutination, India ink microscopy, and CRAG LFA for 832 HIV-infected persons with suspected meningitis during 2006-2009 (n = 299) in Uganda and during 2010-2012 (n = 533) in Uganda and South Africa. CRAG LFA had the best performance (sensitivity 99.3%, specificity 99.1%). Culture sensitivity was dependent on CSF volume (82.4% for 10 μL, 94.2% for 100 μL). CRAG latex agglutination test sensitivity (97.0%-97.8%) and specificity (85.9%-100%) varied between manufacturers. India ink microscopy was 86% sensitive. Laser thermal contrast had 92% accuracy (R = 0.91, p<0.001) in quantifying CRAG titers from 1 LFA strip to within <1.5 dilutions of actual CRAG titers. CRAG LFA is a major advance for meningitis diagnostics in resource-limited settings.
Keywords: Cryptococcus spp.; HIV; India ink microscopy; South Africa; Uganda; cerebrospinal fluid; cryptococcal antigen; cryptococcal antigen latex agglutination; cryptococcal meningitis; culture; diagnostic techniques; fungi; laser thermal contrast measurement; lateral flow immunochromatographic assay; point-of-care systems; sensitivity; specificity; validation studies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

