Evaluation of DNA primase DnaG as a potential target for antibiotics
- PMID: 24379196
- PMCID: PMC3957862
- DOI: 10.1128/AAC.01721-13
Evaluation of DNA primase DnaG as a potential target for antibiotics
Abstract
Mycobacteria contain genes for several DNA-dependent RNA primases, including dnaG, which encodes an essential replication enzyme that has been proposed as a target for antituberculosis compounds. An in silico analysis revealed that mycobacteria also possess archaeo-eukaryotic superfamily primases (AEPs) of unknown function. Using a homologous recombination system, we obtained direct evidence that wild-type dnaG cannot be deleted from the chromosome of Mycobacterium smegmatis without disrupting viability, even in backgrounds in which mycobacterial AEPs are overexpressed. In contrast, single-deletion AEP mutants or mutants defective for all four identified M. smegmatis AEP genes did not exhibit growth defects under standard laboratory conditions. Deletion of native dnaG in M. smegmatis was tolerated only after the integration of an extra intact copy of the M. smegmatis or Mycobacterium tuberculosis dnaG gene, under the control of chemically inducible promoters, into the attB site of the chromosome. M. tuberculosis and M. smegmatis DnaG proteins were overproduced and purified, and their primase activities were confirmed using radioactive RNA synthesis assays. The enzymes appeared to be sensitive to known inhibitors (suramin and doxorubicin) of DnaG. Notably, M. smegmatis bacilli appeared to be sensitive to doxorubicin and resistant to suramin. The growth and survival of conditional mutant mycobacterial strains in which DnaG was significantly depleted were only slightly affected under standard laboratory conditions. Thus, although DnaG is essential for mycobacterial viability, only low levels of protein are required for growth. This suggests that very efficient inhibition of enzyme activity would be required for mycobacterial DnaG to be useful as an antibiotic target.
Figures
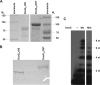
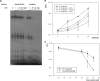

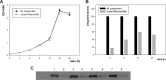
Similar articles
-
A novel non-radioactive primase-pyrophosphatase activity assay and its application to the discovery of inhibitors of Mycobacterium tuberculosis primase DnaG.Nucleic Acids Res. 2013 Feb 1;41(4):e56. doi: 10.1093/nar/gks1292. Epub 2012 Dec 24. Nucleic Acids Res. 2013. PMID: 23267008 Free PMC article.
-
Antimycobacterial activity of DNA intercalator inhibitors of Mycobacterium tuberculosis primase DnaG.J Antibiot (Tokyo). 2015 Mar;68(3):153-7. doi: 10.1038/ja.2014.131. Epub 2014 Sep 24. J Antibiot (Tokyo). 2015. PMID: 25248725 Free PMC article.
-
Evaluation of NAD(+) -dependent DNA ligase of mycobacteria as a potential target for antibiotics.Antimicrob Agents Chemother. 2007 Aug;51(8):2888-97. doi: 10.1128/AAC.00254-07. Epub 2007 Jun 4. Antimicrob Agents Chemother. 2007. PMID: 17548501 Free PMC article.
-
Potential drug targets in the Mycobacterium tuberculosis cytochrome P450 system.J Inorg Biochem. 2018 Mar;180:235-245. doi: 10.1016/j.jinorgbio.2018.01.010. Epub 2018 Jan 12. J Inorg Biochem. 2018. PMID: 29352597 Free PMC article. Review.
-
DnaG Primase-A Target for the Development of Novel Antibacterial Agents.Antibiotics (Basel). 2018 Aug 13;7(3):72. doi: 10.3390/antibiotics7030072. Antibiotics (Basel). 2018. PMID: 30104489 Free PMC article. Review.
Cited by
-
Targeting DNA Replication and Repair for the Development of Novel Therapeutics against Tuberculosis.Front Mol Biosci. 2017 Nov 14;4:75. doi: 10.3389/fmolb.2017.00075. eCollection 2017. Front Mol Biosci. 2017. PMID: 29184888 Free PMC article. Review.
-
Discovery of disease-adapted bacterial lineages in inflammatory bowel diseases.Cell Host Microbe. 2024 Jul 10;32(7):1147-1162.e12. doi: 10.1016/j.chom.2024.05.022. Epub 2024 Jun 24. Cell Host Microbe. 2024. PMID: 38917808 Free PMC article.
-
Cobalamin is present in cells of non-tuberculous mycobacteria, but not in Mycobacterium tuberculosis.Sci Rep. 2021 Jun 10;11(1):12267. doi: 10.1038/s41598-021-91430-w. Sci Rep. 2021. PMID: 34112827 Free PMC article.
-
RNase HI Is Essential for Survival of Mycobacterium smegmatis.PLoS One. 2015 May 12;10(5):e0126260. doi: 10.1371/journal.pone.0126260. eCollection 2015. PLoS One. 2015. PMID: 25965344 Free PMC article.
-
Conditional Silencing by CRISPRi Reveals the Role of DNA Gyrase in Formation of Drug-Tolerant Persister Population in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2019 Mar 26;9:70. doi: 10.3389/fcimb.2019.00070. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30972304 Free PMC article.
References
-
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. 2009. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136:420–425. 10.1378/chest.08-2427 - DOI - PubMed
-
- Centers for Disease Control and Prevention 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs worldwide, 2000-2004. MMWR Morb. Mortal. Wkly. Rep. 55:301–305 - PubMed
-
- Raviglione M. 2006. XDR-TB: entering the post-antibiotic era? Int. J. Tuberc. Lung Dis. 10:1185–1187 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

