In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor
- PMID: 24379202
- PMCID: PMC3957832
- DOI: 10.1128/AAC.02403-13
In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor
Abstract
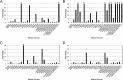
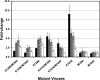
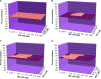
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are a mainstay of therapy for treating human immunodeficiency type 1 virus (HIV-1)-infected patients. MK-1439 is a novel NNRTI with a 50% inhibitory concentration (IC50) of 12, 9.7, and 9.7 nM against the wild type (WT) and K103N and Y181C reverse transcriptase (RT) mutants, respectively, in a biochemical assay. Selectivity and cytotoxicity studies confirmed that MK-1439 is a highly specific NNRTI with minimum off-target activities. In the presence of 50% normal human serum (NHS), MK-1439 showed excellent potency in suppressing the replication of WT virus, with a 95% effective concentration (EC95) of 20 nM, as well as K103N, Y181C, and K103N/Y181C mutant viruses with EC95 of 43, 27, and 55 nM, respectively. MK-1439 exhibited similar antiviral activities against 10 different HIV-1 subtype viruses (a total of 93 viruses). In addition, the susceptibility of a broader array of clinical NNRTI-associated mutant viruses (a total of 96 viruses) to MK-1439 and other benchmark NNRTIs was investigated. The results showed that the mutant profile of MK-1439 was superior overall to that of efavirenz (EFV) and comparable to that of etravirine (ETR) and rilpivirine (RPV). Furthermore, E138K, Y181C, and K101E mutant viruses that are associated with ETR and RPV were susceptible to MK-1439 with a fold change (FC) of <3. A two-drug in vitro combination study indicated that MK-1439 acts nonantagonistically in the antiviral activity with each of 18 FDA-licensed drugs for HIV infection. Taken together, these in vitro data suggest that MK-1439 possesses the desired properties for further development as a new antiviral agent.
Figures



References
-
- Hopkins AL, Ren J, Esnouf RM, Willcox BE, Jones EY, Ross C, Miyasaka T, Walker RT, Tanaka H, Stammers DK, Stuart DI. 1996. Complexes of HIV-1 reverse transcriptase with inhibitors of the HEPT series reveal conformational changes relevant to the design of potent non-nucleoside inhibitors. J. Med. Chem. 39:1589–1600. 10.1021/jm960056x - DOI - PubMed
-
- Ambrose Z, Julias JG, Boyer PL, Kewalramani VN, Hughes SH. 2006. The level of reverse transcriptase (RT) in human immunodeficiency virus type 1 particles affects susceptibility to nonnucleoside RT inhibitors but not to lamivudine. J. Virol. 80:2578–2581. 10.1128/JVI.80.5.2578-2581.2006 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

