Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract
- PMID: 24379203
- PMCID: PMC3957845
- DOI: 10.1128/AAC.02031-13
Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract
Abstract
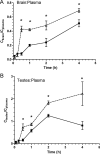
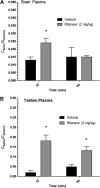
The blood-testis barrier and blood-brain barrier are responsible for protecting the male genital tract and central nervous system from xenobiotic exposure. In HIV-infected patients, low concentrations of antiretroviral drugs in cerebrospinal fluid and seminal fluid have been reported. One mechanism that may contribute to reduced concentrations is the expression of ATP-binding cassette drug efflux transporters, such as P-glycoprotein (P-gp). The objective of this study was to investigate in vivo the tissue distribution of the HIV protease inhibitor atazanavir in wild-type (WT) mice, P-gp/breast cancer resistance protein (Bcrp)-knockout (Mdr1a-/-, Mdr1b-/-, and Abcg2-/- triple-knockout [TKO]) mice, and Cyp3a-/- (Cyp) mice. WT mice and Cyp mice were pretreated with a P-gp/Bcrp inhibitor, elacridar (5 mg/kg of body weight), and the HIV protease inhibitor and boosting agent ritonavir (2 mg/kg intravenously [i.v.]), respectively. Atazanavir (10 mg/kg) was administered i.v. Atazanavir concentrations in plasma (Cplasma), brain (Cbrain), and testes (Ctestes) were quantified at various times by liquid chromatography-tandem mass spectrometry. In TKO mice, we demonstrated a significant increase in atazanavir Cbrain/Cplasma (5.4-fold) and Ctestes/Cplasma (4.6-fold) ratios compared to those in WT mice (P<0.05). Elacridar-treated WT mice showed a significant increase in atazanavir Cbrain/Cplasma (12.3-fold) and Ctestes/Cplasma (13.5-fold) ratios compared to those in vehicle-treated WT mice. In Cyp mice pretreated with ritonavir, significant (P<0.05) increases in atazanavir Cbrain/Cplasma (1.8-fold) and Ctestes/Cplasma (9.5-fold) ratios compared to those in vehicle-treated WT mice were observed. These data suggest that drug efflux transporters, i.e., P-gp, are involved in limiting the ability of atazanavir to permeate the rodent brain and genital tract. Since these transporters are known to be expressed in humans, they could contribute to the low cerebrospinal and seminal fluid antiretroviral concentrations reported in the clinic.
Figures



Similar articles
-
Influence of atazanavir 200 mg on the intracellular and plasma pharmacokinetics of saquinavir and ritonavir 1600/100 mg administered once daily in HIV-infected patients.J Antimicrob Chemother. 2006 Nov;58(5):1009-16. doi: 10.1093/jac/dkl379. Epub 2006 Sep 19. J Antimicrob Chemother. 2006. PMID: 16984898
-
Comparison of ABC transporter modulation by atazanavir in lymphocytes and human brain endothelial cells: ABC transporters are involved in the atazanavir-limited passage across an in vitro human model of the blood-brain barrier.AIDS Res Hum Retroviruses. 2008 Sep;24(9):1147-54. doi: 10.1089/aid.2007.0022. AIDS Res Hum Retroviruses. 2008. PMID: 18729774
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
-
Tenofovir comedication does not impair the steady-state pharmacokinetics of ritonavir-boosted atazanavir in HIV-1-infected adults.Eur J Clin Pharmacol. 2007 Oct;63(10):935-40. doi: 10.1007/s00228-007-0344-y. Epub 2007 Jul 31. Eur J Clin Pharmacol. 2007. PMID: 17665183 Clinical Trial.
-
Atazanavir/ritonavir: a review of its use in HIV therapy.Drugs Today (Barc). 2008 Feb;44(2):103-32. doi: 10.1358/dot.2008.44.2.1137107. Drugs Today (Barc). 2008. PMID: 18389089 Review.
Cited by
-
An update on drug-drug interactions between antiretroviral therapies and drugs of abuse in HIV systems.Expert Opin Drug Metab Toxicol. 2020 Nov;16(11):1005-1018. doi: 10.1080/17425255.2020.1814737. Epub 2020 Aug 31. Expert Opin Drug Metab Toxicol. 2020. PMID: 32842791 Free PMC article. Review.
-
Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions.Pharmacol Rev. 2018 Apr;70(2):278-314. doi: 10.1124/pr.117.014647. Pharmacol Rev. 2018. PMID: 29496890 Free PMC article. Review.
-
Testis Is a Sanctuary Site for HIV-1.Adv Exp Med Biol. 2025;1469:433-440. doi: 10.1007/978-3-031-82990-1_19. Adv Exp Med Biol. 2025. PMID: 40301268 Review.
-
Semen as the Way Forward to Understand HIV-1 Transmission.J Infect Dis. 2016 Nov 15;214(10):1473-1474. doi: 10.1093/infdis/jiw409. Epub 2016 Aug 30. J Infect Dis. 2016. PMID: 27578846 Free PMC article. No abstract available.
-
Therapeutic Potential and Utility of Elacridar with Respect to P-glycoprotein Inhibition: An Insight from the Published In Vitro, Preclinical and Clinical Studies.Eur J Drug Metab Pharmacokinet. 2017 Dec;42(6):915-933. doi: 10.1007/s13318-017-0411-4. Eur J Drug Metab Pharmacokinet. 2017. PMID: 28374336 Review.
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents 2013. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. February 2012. U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
-
- Stebbing J, Bower M, Mandalia S, Nelson M, Gazzard B. 2006. Highly active anti-retroviral therapy (HAART)-induced maintenance of adaptive but not innate immune parameters is associated with protection from HIV-induced mortality. Clin. Exp. Immunol. 145:271–276. 10.1111/j.1365-2249.2006.03147.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

