New β-lactamase inhibitors: a therapeutic renaissance in an MDR world
- PMID: 24379206
- PMCID: PMC4023773
- DOI: 10.1128/AAC.00826-13
New β-lactamase inhibitors: a therapeutic renaissance in an MDR world
Abstract
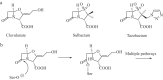
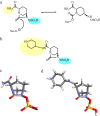
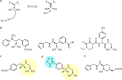
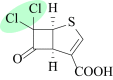
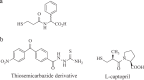
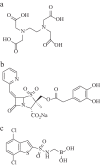
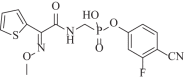
As the incidence of Gram-negative bacterial infections for which few effective treatments remain increases, so does the contribution of drug-hydrolyzing β-lactamase enzymes to this serious clinical problem. This review highlights recent advances in β-lactamase inhibitors and focuses on agents with novel mechanisms of action against a wide range of enzymes. To this end, we review the β-lactamase inhibitors currently in clinical trials, select agents still in preclinical development, and older therapeutic approaches that are being revisited. Particular emphasis is placed on the activity of compounds at the forefront of the developmental pipeline, including the diazabicyclooctane inhibitors (avibactam and MK-7655) and the boronate RPX7009. With its novel reversible mechanism, avibactam stands to be the first new β-lactamase inhibitor brought into clinical use in the past 2 decades. Our discussion includes the importance of selecting the appropriate partner β-lactam and dosing regimens for these promising agents. This "renaissance" of β-lactamase inhibitors offers new hope in a world plagued by multidrug-resistant (MDR) Gram-negative bacteria.
Figures
Similar articles
-
Ceftazidime-Avibactam: A Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms.Clin Ther. 2016 Mar;38(3):431-44. doi: 10.1016/j.clinthera.2016.01.018. Epub 2016 Mar 2. Clin Ther. 2016. PMID: 26948862 Review.
-
Ceftolozane/tazobactam and ceftazidime/avibactam: two novel β-lactam/β-lactamase inhibitor combination agents for the treatment of resistant Gram-negative bacterial infections.Int J Antimicrob Agents. 2015 Sep;46(3):266-71. doi: 10.1016/j.ijantimicag.2015.05.003. Epub 2015 Jun 14. Int J Antimicrob Agents. 2015. PMID: 26143591 Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
Novel β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant Gram-negative pathogens.Expert Opin Drug Metab Toxicol. 2019 Feb;15(2):133-149. doi: 10.1080/17425255.2019.1563071. Epub 2019 Jan 10. Expert Opin Drug Metab Toxicol. 2019. PMID: 30626244 Review.
-
Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections.J Antimicrob Chemother. 2016 Oct;71(10):2713-22. doi: 10.1093/jac/dkw239. Epub 2016 Jul 17. J Antimicrob Chemother. 2016. PMID: 27432599 Review.
Cited by
-
Drug resistance phenotypes and genotypes in Mexico in representative gram-negative species: Results from the infivar network.PLoS One. 2021 Mar 17;16(3):e0248614. doi: 10.1371/journal.pone.0248614. eCollection 2021. PLoS One. 2021. PMID: 33730101 Free PMC article.
-
Effect of the β-Lactamase Inhibitor Vaborbactam Combined with Meropenem against Serine Carbapenemase-Producing Enterobacteriaceae.Antimicrob Agents Chemother. 2016 Aug 22;60(9):5454-8. doi: 10.1128/AAC.00711-16. Print 2016 Sep. Antimicrob Agents Chemother. 2016. PMID: 27381386 Free PMC article.
-
The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia.Ann Clin Microbiol Antimicrob. 2017 Jan 6;16(1):1. doi: 10.1186/s12941-016-0177-6. Ann Clin Microbiol Antimicrob. 2017. PMID: 28061852 Free PMC article.
-
Next-generation approaches to understand and combat the antibiotic resistome.Nat Rev Microbiol. 2017 Jul;15(7):422-434. doi: 10.1038/nrmicro.2017.28. Epub 2017 Apr 10. Nat Rev Microbiol. 2017. PMID: 28392565 Free PMC article. Review.
-
Identification of Potential Inhibitors of Three NDM Variants of Klebsiella Species from Natural Compounds: A Molecular Docking, Molecular Dynamics Simulation and MM-PBSA Study.Curr Comput Aided Drug Des. 2025;21(2):142-165. doi: 10.2174/0115734099294294240311061115. Curr Comput Aided Drug Des. 2025. PMID: 38504567
References
-
- Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. 2011. Tackling antibiotic resistance. Nat. Rev. Microbiol. 9:894–896. 10.1038/nrmicro2693 - DOI - PMC - PubMed
-
- Fisher J, Mobashery S. 2010. Enzymology of antibacterial resistance, p 443–487 In Mander L, Liu H. (ed), Comprehensive natural products chemistry II. Elsevier, Oxford, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

