EDD enhances cell survival and cisplatin resistance and is a therapeutic target for epithelial ovarian cancer
- PMID: 24379240
- PMCID: PMC4004201
- DOI: 10.1093/carcin/bgt489
EDD enhances cell survival and cisplatin resistance and is a therapeutic target for epithelial ovarian cancer
Abstract
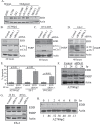
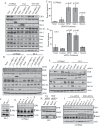
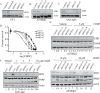
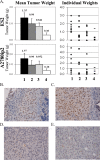
The E3 ubiquitin ligase EDD is overexpressed in recurrent, platinum-resistant ovarian cancers, suggesting a role in tumor survival and/or platinum resistance. EDD knockdown by small interfering RNA (siRNA) induced apoptosis in A2780ip2, OVCAR5 and ES-2 ovarian cancer cells, correlating with loss of the prosurvival protein myeloid cell leukemia sequence 1 (Mcl-1) through a glycogen synthase kinase 3 beta-independent mechanism. SiRNA to EDD or Mcl-1 induced comparable levels of apoptosis in A2780ip2 and ES-2 cells. Stable overexpression of Mcl-1 protected cells from apoptosis following EDD knockdown, accompanied by a loss of endogenous, but not exogenous, Mcl-1 protein, suggesting that EDD regulated Mcl-1 synthesis. Indeed, EDD knockdown induced a 1.87-fold decrease in Mcl-1 messenger RNA and EDD transfection enhanced murine Mcl-1 promoter-driven luciferase expression 5-fold. To separate EDD survival and potential cisplatin resistance functions, we generated EDD shRNA stable cell lines that could survive initial EDD knockdown and showed that these cells were 4- to 21-fold more sensitive to cisplatin. Moreover, transient EDD overexpression in COS-7 cells was sufficient to promote cisplatin resistance 2.4-fold, dependent upon its E3 ligase activity. In vivo, mouse intraperitoneal ES-2 and A2780ip2 xenograft experiments showed that mice treated with EDD siRNA by nanoliposomal delivery [1,2-dioleoyl-sn-glycero-3-phophatidylcholine (DOPC)] and cisplatin had significantly less tumor burden than those treated with control siRNA/DOPC alone (ES-2, 77.9% reduction, P = 0.004; A2780ip2, 75.9% reduction, P = 0.042) or control siRNA/DOPC with cisplatin in ES-2 (64.4% reduction, P = 0.035), with a trend in A2780ip2 (60.3% reduction, P = 0.168). These results identify EDD as a dual regulator of cell survival and cisplatin resistance and suggest that EDD is a therapeutic target for ovarian cancer.
Figures


Similar articles
-
MCT1 promotes the cisplatin-resistance by antagonizing Fas in epithelial ovarian cancer.Int J Clin Exp Pathol. 2015 Mar 1;8(3):2710-8. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26045776 Free PMC article.
-
The E3 ubiquitin ligase EDD is an adverse prognostic factor for serous epithelial ovarian cancer and modulates cisplatin resistance in vitro.Br J Cancer. 2008 Mar 25;98(6):1085-93. doi: 10.1038/sj.bjc.6604281. Epub 2008 Mar 18. Br J Cancer. 2008. PMID: 18349819 Free PMC article.
-
HOTAIR is a potential target for the treatment of cisplatin‑resistant ovarian cancer.Mol Med Rep. 2015 Aug;12(2):2211-6. doi: 10.3892/mmr.2015.3562. Epub 2015 Mar 27. Mol Med Rep. 2015. PMID: 25824616
-
Deregulated circRNAs in Epithelial Ovarian Cancer With Activity in Preclinical In Vivo Models: Identification of Targets and New Modalities for Therapeutic Intervention.Cancer Genomics Proteomics. 2024 Apr 26;21(3):213-237. doi: 10.21873/cgp.20442. Cancer Genomics Proteomics. 2024. PMID: 38670587 Free PMC article. Review.
-
Epigenetic mechanisms and therapeutic targets of chemotherapy resistance in epithelial ovarian cancer.Ann Med. 2015;47(5):359-69. doi: 10.3109/07853890.2015.1043140. Epub 2015 Jul 10. Ann Med. 2015. PMID: 26158617 Review.
Cited by
-
E3 ubiquitin ligase isolated by differential display regulates cervical cancer growth in vitro and in vivo via microRNA-143.Exp Ther Med. 2016 Aug;12(2):676-682. doi: 10.3892/etm.2016.3429. Epub 2016 Jun 6. Exp Ther Med. 2016. PMID: 27446260 Free PMC article.
-
MOAP-1, UBR5 and cisplatin resistance in ovarian cancer.Transl Cancer Res. 2017 Feb;6(Suppl 1):S18-S21. doi: 10.21037/tcr.2017.02.01. Transl Cancer Res. 2017. PMID: 29607295 Free PMC article. No abstract available.
-
E3 Ubiquitin Ligase in Anticancer Drugdsla Resistance: Recent Advances and Future Potential.Front Pharmacol. 2021 Apr 15;12:645864. doi: 10.3389/fphar.2021.645864. eCollection 2021. Front Pharmacol. 2021. PMID: 33935743 Free PMC article. Review.
-
Novel players in the development of chemoresistance in ovarian cancer: ovarian cancer stem cells, non-coding RNA and nuclear receptors.Cancer Drug Resist. 2024 Feb 28;7:6. doi: 10.20517/cdr.2023.152. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38434767 Free PMC article. Review.
-
Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy.Cancers (Basel). 2022 Apr 29;14(9):2220. doi: 10.3390/cancers14092220. Cancers (Basel). 2022. PMID: 35565348 Free PMC article. Review.
References
-
- Henderson M.J., et al. (2002). EDD, the human hyperplastic discs protein, has a role in progesterone receptor coactivation and potential involvement in DNA damage response. J. Biol. Chem., 277, 26468–26478 - PubMed
-
- Bethard J.R., et al. (2011). Identification of phosphorylation sites on the E3 ubiquitin ligase UBR5/EDD. J. Proteomics, 75, 603–609 - PubMed
-
- Eblen S.T., et al. (2003). Identification of novel ERK2 substrates through use of an engineered kinase and ATP analogs. J. Biol. Chem., 278, 14926–14935 - PubMed
-
- Rechsteiner M., et al. (2005). Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol., 15, 27–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

