The inner rod protein controls substrate switching and needle length in a Salmonella type III secretion system
- PMID: 24379359
- PMCID: PMC3896146
- DOI: 10.1073/pnas.1319698111
The inner rod protein controls substrate switching and needle length in a Salmonella type III secretion system
Abstract
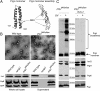
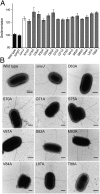
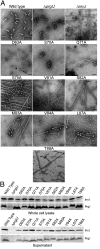
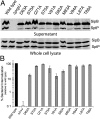
Type III secretion machines are essential for the biology of many bacteria that are pathogenic or symbiotic for animals, plants, or insects. They exert their function by delivering bacterial effector proteins into target eukaryotic cells. The core component of these machines is the needle complex, a multiprotein structure that spans the bacterial envelope and serves as a conduit for proteins that transit this secretion pathway. The needle complex is composed of a multiring base embedded in the bacterial envelope and a filament-like structure, the needle, that projects from the bacterial surface and is linked to the base by the inner rod. Assembly of the needle complex proceeds in a step-wise fashion that is initiated by the assembly of the base and is followed by the export of the building subunits for the needle and inner rod substructures. Once assembled, the needle complex reprograms its specificity and becomes competent for the secretion of effector proteins. Here through genetic, biochemical, and electron microscopy analyses of the Salmonella inner rod protein subunit PrgJ we present evidence that the assembly of the inner rod dictates the timing of substrate switching and needle length. Furthermore, the identification of mutations in PrgJ that specifically alter the hierarchy of protein secretion provides additional support for a complex role of the inner rod substructure in type III secretion.
Keywords: Salmonella pathogenesis; bacterial pathogenesis; organelle assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Determination of the Stoichiometry of the Complete Bacterial Type III Secretion Needle Complex Using a Combined Quantitative Proteomic Approach.Mol Cell Proteomics. 2016 May;15(5):1598-609. doi: 10.1074/mcp.M115.056598. Epub 2016 Feb 21. Mol Cell Proteomics. 2016. PMID: 26900162 Free PMC article.
-
Role of autocleavage in the function of a type III secretion specificity switch protein in Salmonella enterica serovar Typhimurium.mBio. 2015 Oct 13;6(5):e01459-15. doi: 10.1128/mBio.01459-15. mBio. 2015. PMID: 26463164 Free PMC article.
-
A polymorphic helix of a Salmonella needle protein relays signals defining distinct steps in type III secretion.PLoS Biol. 2019 Jul 1;17(7):e3000351. doi: 10.1371/journal.pbio.3000351. eCollection 2019 Jul. PLoS Biol. 2019. PMID: 31260457 Free PMC article.
-
Assembly of the bacterial type III secretion machinery.FEMS Microbiol Rev. 2014 Jul;38(4):802-22. doi: 10.1111/1574-6976.12061. Epub 2014 Feb 17. FEMS Microbiol Rev. 2014. PMID: 24484471 Review.
-
The type III secretion injectisome, a complex nanomachine for intracellular 'toxin' delivery.Biol Chem. 2010 Jul;391(7):745-51. doi: 10.1515/BC.2010.079. Biol Chem. 2010. PMID: 20482311 Review.
Cited by
-
Chaperone-mediated secretion switching from early to middle substrates in the type III secretion system encoded by Salmonella pathogenicity island 2.J Biol Chem. 2019 Mar 8;294(10):3783-3793. doi: 10.1074/jbc.RA118.005072. Epub 2019 Jan 16. J Biol Chem. 2019. PMID: 30651351 Free PMC article.
-
Assembly, structure, function and regulation of type III secretion systems.Nat Rev Microbiol. 2017 Jun;15(6):323-337. doi: 10.1038/nrmicro.2017.20. Epub 2017 Apr 10. Nat Rev Microbiol. 2017. PMID: 28392566 Review.
-
Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells.Annu Rev Microbiol. 2014;68:415-38. doi: 10.1146/annurev-micro-092412-155725. Epub 2014 Jun 18. Annu Rev Microbiol. 2014. PMID: 25002086 Free PMC article. Review.
-
Determination of the Stoichiometry of the Complete Bacterial Type III Secretion Needle Complex Using a Combined Quantitative Proteomic Approach.Mol Cell Proteomics. 2016 May;15(5):1598-609. doi: 10.1074/mcp.M115.056598. Epub 2016 Feb 21. Mol Cell Proteomics. 2016. PMID: 26900162 Free PMC article.
-
Type III-Dependent Translocation of HrpB2 by a Nonpathogenic hpaABC Mutant of the Plant-Pathogenic Bacterium Xanthomonas campestris pv. vesicatoria.Appl Environ Microbiol. 2016 May 16;82(11):3331-3347. doi: 10.1128/AEM.00537-16. Print 2016 Jun 1. Appl Environ Microbiol. 2016. PMID: 27016569 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

