Rapid disorganization of mechanically interacting systems of mammary acini
- PMID: 24379367
- PMCID: PMC3896145
- DOI: 10.1073/pnas.1311312110
Rapid disorganization of mechanically interacting systems of mammary acini
Abstract
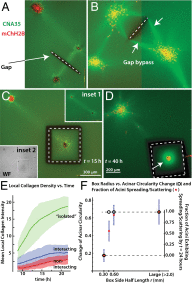
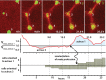
Cells and multicellular structures can mechanically align and concentrate fibers in their ECM environment and can sense and respond to mechanical cues by differentiating, branching, or disorganizing. Here we show that mammary acini with compromised structural integrity can interconnect by forming long collagen lines. These collagen lines then coordinate and accelerate transition to an invasive phenotype. Interacting acini begin to disorganize within 12.5 ± 4.7 h in a spatially coordinated manner, whereas acini that do not interact mechanically with other acini disorganize more slowly (in 21.8 ± 4.1 h) and to a lesser extent (P < 0.0001). When the directed mechanical connections between acini were cut with a laser, the acini reverted to a slowly disorganizing phenotype. When acini were fully mechanically isolated from other acini and also from the bulk gel by box-cuts with a side length <900 μm, transition to an invasive phenotype was blocked in 20 of 20 experiments, regardless of waiting time. Thus, pairs or groups of mammary acini can interact mechanically over long distances through the collagen matrix, and these directed mechanical interactions facilitate transition to an invasive phenotype.
Keywords: cancer; mechanobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cell Force-Driven Basement Membrane Disruption Fuels EGF- and Stiffness-Induced Invasive Cell Dissemination from Benign Breast Gland Acini.Int J Mol Sci. 2021 Apr 12;22(8):3962. doi: 10.3390/ijms22083962. Int J Mol Sci. 2021. PMID: 33921304 Free PMC article.
-
Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):163-8. doi: 10.1073/pnas.1201141110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248267 Free PMC article.
-
Building bridges toward invasion: tumor promoter treatment induces a novel protein kinase C-dependent phenotype in MCF10A mammary cell acini.PLoS One. 2014 Mar 5;9(3):e90722. doi: 10.1371/journal.pone.0090722. eCollection 2014. PLoS One. 2014. PMID: 24599099 Free PMC article.
-
Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression.Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a003228. doi: 10.1101/cshperspect.a003228. Cold Spring Harb Perspect Biol. 2011. PMID: 20980442 Free PMC article. Review.
-
Modeling mammary organogenesis from biological first principles: Cells and their physical constraints.Prog Biophys Mol Biol. 2016 Oct;122(1):58-69. doi: 10.1016/j.pbiomolbio.2016.08.004. Epub 2016 Aug 18. Prog Biophys Mol Biol. 2016. PMID: 27544910 Free PMC article. Review.
Cited by
-
Local extensional flows promote long-range fiber alignment in 3D collagen hydrogels.Biofabrication. 2022 Jun 23;14(3):10.1088/1758-5090/ac7824. doi: 10.1088/1758-5090/ac7824. Biofabrication. 2022. PMID: 35735228 Free PMC article.
-
Growth kinetics and power laws indicate distinct mechanisms of cell-cell interactions in the aggregation process.Biophys J. 2022 Feb 1;121(3):481-490. doi: 10.1016/j.bpj.2021.12.030. Epub 2021 Dec 28. Biophys J. 2022. PMID: 34968426 Free PMC article.
-
The portal fibroblast: not just a poor man's stellate cell.Gastroenterology. 2014 Jul;147(1):41-7. doi: 10.1053/j.gastro.2014.05.001. Epub 2014 May 9. Gastroenterology. 2014. PMID: 24814904 Free PMC article. Review.
-
Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs.Proc Natl Acad Sci U S A. 2016 Dec 6;113(49):14043-14048. doi: 10.1073/pnas.1613058113. Epub 2016 Nov 21. Proc Natl Acad Sci U S A. 2016. PMID: 27872289 Free PMC article.
-
Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma.Mol Cancer Res. 2016 Mar;14(3):287-95. doi: 10.1158/1541-7786.MCR-15-0307. Epub 2015 Dec 2. Mol Cancer Res. 2016. PMID: 26631572 Free PMC article.
References
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. - PubMed
-
- Rauzi M, Lecuit T. Closing in on mechanisms of tissue morphogenesis. Cell. 2009;137(7):1183–1185. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. - PubMed
-
- Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006;20(7):811–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

