Mycothiol/mycoredoxin 1-dependent reduction of the peroxiredoxin AhpE from Mycobacterium tuberculosis
- PMID: 24379404
- PMCID: PMC3931079
- DOI: 10.1074/jbc.M113.510248
Mycothiol/mycoredoxin 1-dependent reduction of the peroxiredoxin AhpE from Mycobacterium tuberculosis
Abstract
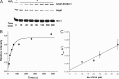
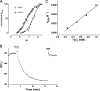
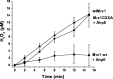
Mycobacterium tuberculosis (M. tuberculosis), the pathogen responsible for tuberculosis, detoxifies cytotoxic peroxides produced by activated macrophages. M. tuberculosis expresses alkyl hydroxyperoxide reductase E (AhpE), among other peroxiredoxins. So far the system that reduces AhpE was not known. We identified M. tuberculosis mycoredoxin-1 (MtMrx1) acting in combination with mycothiol and mycothiol disulfide reductase (MR), as a biologically relevant reducing system for MtAhpE. MtMrx1, a glutaredoxin-like, mycothiol-dependent oxidoreductase, directly reduces the oxidized form of MtAhpE, through a protein mixed disulfide with the N-terminal cysteine of MtMrx1 and the sulfenic acid derivative of the peroxidatic cysteine of MtAhpE. This disulfide is then reduced by the C-terminal cysteine in MtMrx1. Accordingly, MtAhpE catalyzes the oxidation of wt MtMrx1 by hydrogen peroxide but not of MtMrx1 lacking the C-terminal cysteine, confirming a dithiolic mechanism. Alternatively, oxidized MtAhpE forms a mixed disulfide with mycothiol, which in turn is reduced by MtMrx1 using a monothiolic mechanism. We demonstrated the H2O2-dependent NADPH oxidation catalyzed by MtAhpE in the presence of MR, Mrx1, and mycothiol. Disulfide formation involving mycothiol probably competes with the direct reduction by MtMrx1 in aqueous intracellular media, where mycothiol is present at millimolar concentrations. However, MtAhpE was found to be associated with the membrane fraction, and since mycothiol is hydrophilic, direct reduction by MtMrx1 might be favored. The results reported herein allow the rationalization of peroxide detoxification actions inferred for mycothiol, and more recently, for Mrx1 in cellular systems. We report the first molecular link between a thiol-dependent peroxidase and the mycothiol/Mrx1 pathway in Mycobacteria.
Keywords: Hydrogen Peroxide; Mycobacterium tuberculosis; Mycoredoxin; Mycothiol; Peroxiredoxin; Redox Signaling; Thiol.
Figures




Similar articles
-
Redox chemistry of Mycobacterium tuberculosis alkylhydroperoxide reductase E (AhpE): Structural and mechanistic insight into a mycoredoxin-1 independent reductive pathway of AhpE via mycothiol.Free Radic Biol Med. 2016 Aug;97:588-601. doi: 10.1016/j.freeradbiomed.2016.07.007. Epub 2016 Jul 12. Free Radic Biol Med. 2016. PMID: 27417938
-
Kinetics of formation and reactivity of the persulfide in the one-cysteine peroxiredoxin from Mycobacterium tuberculosis.J Biol Chem. 2019 Sep 13;294(37):13593-13605. doi: 10.1074/jbc.RA119.008883. Epub 2019 Jul 16. J Biol Chem. 2019. PMID: 31311857 Free PMC article.
-
Thiol and sulfenic acid oxidation of AhpE, the one-cysteine peroxiredoxin from Mycobacterium tuberculosis: kinetics, acidity constants, and conformational dynamics.Biochemistry. 2009 Oct 13;48(40):9416-26. doi: 10.1021/bi901221s. Biochemistry. 2009. PMID: 19737009
-
Chemistry and Redox Biology of Mycothiol.Antioxid Redox Signal. 2018 Feb 20;28(6):487-504. doi: 10.1089/ars.2017.7074. Epub 2017 May 10. Antioxid Redox Signal. 2018. PMID: 28372502 Review.
-
Mycothiol-dependent proteins in actinomycetes.FEMS Microbiol Rev. 2007 Apr;31(3):278-92. doi: 10.1111/j.1574-6976.2006.00062.x. Epub 2007 Feb 26. FEMS Microbiol Rev. 2007. PMID: 17286835 Review.
Cited by
-
Relevance of peroxiredoxins in pathogenic microorganisms.Appl Microbiol Biotechnol. 2021 Aug;105(14-15):5701-5717. doi: 10.1007/s00253-021-11360-5. Epub 2021 Jul 14. Appl Microbiol Biotechnol. 2021. PMID: 34258640 Review.
-
European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS).Redox Biol. 2017 Oct;13:94-162. doi: 10.1016/j.redox.2017.05.007. Epub 2017 May 18. Redox Biol. 2017. PMID: 28577489 Free PMC article. Review.
-
Peroxiredoxins wear many hats: Factors that fashion their peroxide sensing personalities.Redox Biol. 2021 Jun;42:101959. doi: 10.1016/j.redox.2021.101959. Epub 2021 Apr 20. Redox Biol. 2021. PMID: 33895094 Free PMC article. Review.
-
Reactive species and pathogen antioxidant networks during phagocytosis.J Exp Med. 2019 Mar 4;216(3):501-516. doi: 10.1084/jem.20181886. Epub 2019 Feb 21. J Exp Med. 2019. PMID: 30792185 Free PMC article. Review.
-
Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance.Antioxidants (Basel). 2020 Apr 26;9(5):361. doi: 10.3390/antiox9050361. Antioxidants (Basel). 2020. PMID: 32357394 Free PMC article. Review.
References
-
- World Health Organization (2012) Global Tuberculosis Report
-
- Nathan C. (2009) Taming tuberculosis: a challenge for science and society. Cell Host Microbe. 5, 220–224 - PubMed
-
- Fang F. C. (2004) Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2, 820–832 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

