Cross talk between CD3 and CD28 is spatially modulated by protein lateral mobility
- PMID: 24379441
- PMCID: PMC3958039
- DOI: 10.1128/MCB.00842-13
Cross talk between CD3 and CD28 is spatially modulated by protein lateral mobility
Abstract
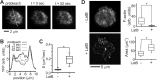
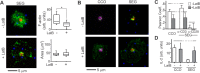
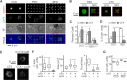
Functional convergence of CD28 costimulation and TCR signaling is critical to T-cell activation and adaptive immunity. These receptors form complex microscale patterns within the immune synapse, although the impact of this spatial organization on cell signaling remains unclear. We investigate this cross talk using micropatterned surfaces that present ligands to these membrane proteins in order to control the organization of signaling molecules within the cell-substrate interface. While primary human CD4(+) T cells were activated by features containing ligands to both CD3 and CD28, this functional convergence was curtailed on surfaces in which engagement of these two systems was separated by micrometer-scale distances. Moreover, phosphorylated Lck was concentrated to regions of CD3 engagement and exhibited a low diffusion rate, suggesting that costimulation is controlled by a balance between the transport of active Lck to CD28 and its deactivation. In support of this model, disruption of the actin cytoskeleton increased Lck mobility and allowed functional T-cell costimulation by spatially separated CD3 and CD28. In primary mouse CD4(+) T cells, a complementary system, reducing the membrane mobility increased the sensitivity to CD3-CD28 separation. These results demonstrate a subcellular reaction-diffusion system that allows cells to sense the microscale organization of the extracellular environment.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
