Sciatic nerve repair with tissue engineered nerve: Olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) conduit in an animal model
- PMID: 24379458
- PMCID: PMC3868134
- DOI: 10.4103/0019-5413.121572
Sciatic nerve repair with tissue engineered nerve: Olfactory ensheathing cells seeded poly(lactic-co-glygolic acid) conduit in an animal model
Abstract
Background and aim: Synthetic nerve conduits have been sought for repair of nerve defects as the autologous nerve grafts causes donor site morbidity and possess other drawbacks. Many strategies have been investigated to improve nerve regeneration through synthetic nerve guided conduits. Olfactory ensheathing cells (OECs) that share both Schwann cell and astrocytic characteristics have been shown to promote axonal regeneration after transplantation. The present study was driven by the hypothesis that tissue-engineered poly(lactic-co-glycolic acid) (PLGA) seeded with OECs would improve peripheral nerve regeneration in a long sciatic nerve defect.
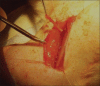
Materials and methods: Sciatic nerve gap of 15 mm was created in six adult female Sprague-Dawley rats and implanted with PLGA seeded with OECs. The nerve regeneration was assessed electrophysiologically at 2, 4 and 6 weeks following implantation. Histopathological examination, scanning electron microscopic (SEM) examination and immunohistochemical analysis were performed at the end of the study.
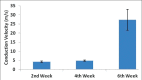
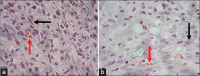
Results: Nerve conduction studies revealed a significant improvement of nerve conduction velocities whereby the mean nerve conduction velocity increases from 4.2 0.4 m/s at week 2 to 27.3 5.7 m/s at week 6 post-implantation (P < 0.0001). Histological analysis revealed presence of spindle-shaped cells. Immunohistochemical analysis further demonstrated the expression of S100 protein in both cell nucleus and the cytoplasm in these cells, hence confirming their Schwann-cell-like property. Under SEM, these cells were found to be actively secreting extracellular matrix.
Conclusion: Tissue-engineered PLGA conduit seeded with OECs provided a permissive environment to facilitate nerve regeneration in a small animal model.
Keywords: Olfactory ensheathing cells; poly(lactic-co-glycolic acid); sciatic nerve defect; tissue engineering.
Conflict of interest statement
Figures


Similar articles
-
PLGA conduit seeded with olfactory ensheathing cells for bridging sciatic nerve defect of rats.J Biomed Mater Res A. 2010 Sep 1;94(3):769-80. doi: 10.1002/jbm.a.32727. J Biomed Mater Res A. 2010. PMID: 20336740
-
The effects of low-intensity ultrasound on peripheral nerve regeneration in poly(DL-lactic acid-co-glycolic acid) conduits seeded with Schwann cells.Ultrasound Med Biol. 2004 Aug;30(8):1079-84. doi: 10.1016/j.ultrasmedbio.2004.06.005. Ultrasound Med Biol. 2004. PMID: 15474752
-
A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration.Tissue Eng. 2000 Apr;6(2):119-27. doi: 10.1089/107632700320748. Tissue Eng. 2000. PMID: 10941207
-
[REGULATORY EFFECT OF OLFACTORY ENSHEATHING CELLS ON INFLAMMATORY CYTOKINES IN REPAIR OF RAT SCIATIC NERVE DEFECT].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1538-1544. doi: 10.7507/1002-1892.20160317. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786348 Chinese.
-
Olfactory-ensheathing cell transplantation for peripheral nerve repair: update on recent developments.Cells Tissues Organs. 2014;200(1):48-58. doi: 10.1159/000369006. Epub 2015 Mar 4. Cells Tissues Organs. 2014. PMID: 25765445 Review.
Cited by
-
Recent Updates in Neuroprotective and Neuroregenerative Potential of Centella asiatica.Malays J Med Sci. 2016 Jan;23(1):4-14. Malays J Med Sci. 2016. PMID: 27540320 Free PMC article. Review.
-
Efficacy of using adipose-derived stem cells and PRP on regeneration of 40 -mm long sciatic nerve defect bridged by polyglycolic-polypropylene mesh in canine model.Stem Cell Res Ther. 2024 Jul 18;15(1):212. doi: 10.1186/s13287-024-03796-z. Stem Cell Res Ther. 2024. PMID: 39020391 Free PMC article.
-
Neuroregenerative effects of olfactory ensheathing cells transplanted in a multi-layered conductive nanofibrous conduit in peripheral nerve repair in rats.J Biomed Sci. 2015 May 20;22(1):35. doi: 10.1186/s12929-015-0144-0. J Biomed Sci. 2015. PMID: 25986461 Free PMC article.
-
Using Stem Cells to Grow Artificial Tissue for Peripheral Nerve Repair.Stem Cells Int. 2016;2016:7502178. doi: 10.1155/2016/7502178. Epub 2016 Apr 26. Stem Cells Int. 2016. PMID: 27212954 Free PMC article. Review.
-
Olfactory ensheathing cells promote nerve regeneration and functional recovery after facial nerve defects.Neural Regen Res. 2019 Jan;14(1):124-131. doi: 10.4103/1673-5374.243717. Neural Regen Res. 2019. PMID: 30531086 Free PMC article.
References
-
- Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–29. - PubMed
-
- Burg K, Thomas C. Tissue engineering. In: Akay M, editor. Wiley Encyclopedia of Biomedical Engineering. New Jersey: John Wiley and Sons; 2006. pp. 3497–512.
-
- Bryan DJ, Holway AH, Wang KK, Silva AE, Trantolo DJ, Wise D, et al. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 2000;6:129–38. - PubMed
-
- Hidayah N, Fadzli A, Ng M, Ruszymah B, Naicker A, Shalimar A, et al. Porous PLGA sheet and acellularized muscle stuffed vein seeded with neural-differentiated MSCs are potential scaffolds for nerve regeneration. Regen Res. 2012;1:1–7.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
