Fullerene-biomolecule conjugates and their biomedicinal applications
- PMID: 24379667
- PMCID: PMC3872219
- DOI: 10.2147/IJN.S52829
Fullerene-biomolecule conjugates and their biomedicinal applications
Abstract
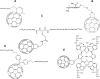
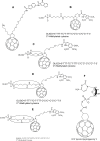
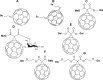
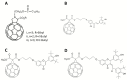
Fullerenes are among the strongest antioxidants and are characterized as "radical sponges." The research on biomedicinal applications of fullerenes has achieved significant progress since the landmark publication by Friedman et al in 1993. Fullerene-biomolecule conjugates have become an important area of research during the past 2 decades. By a thorough literature search, we attempt to update the information about the synthesis of different types of fullerene-biomolecule conjugates, including fullerene-containing amino acids and peptides, oligonucleotides, sugars, and esters. Moreover, we also discuss in this review recently reported data on the biological and pharmaceutical utilities of these compounds and some other fullerene derivatives of biomedical importance. While within the fullerene-biomolecule conjugates, in which fullerene may act as both an antioxidant and a carrier, specific targeting biomolecules conjugated to fullerene will undoubtedly strengthen the delivery of functional fullerenes to sites of clinical interest.
Keywords: amino acid; ester; fullerene; oligonucleotide; peptide; sugar.
Figures


Similar articles
-
Liposomes: versatile and biocompatible nanovesicles for efficient biomolecules delivery.J Nanosci Nanotechnol. 2014 Jan;14(1):755-65. doi: 10.1166/jnn.2014.9080. J Nanosci Nanotechnol. 2014. PMID: 24730295 Review.
-
Fullerene-derivatized amino acids: synthesis, characterization, antioxidant properties, and solid-phase peptide synthesis.Chemistry. 2007;13(9):2530-45. doi: 10.1002/chem.200601186. Chemistry. 2007. PMID: 17236230
-
Effect of phase transition temperature of liposomes on preparation of fullerene-encapsulated liposomes by the fullerene-exchange reaction.Chem Commun (Camb). 2010 Apr 28;46(16):2847-9. doi: 10.1039/b926949e. Epub 2010 Mar 4. Chem Commun (Camb). 2010. PMID: 20369203
-
Medicinal applications of fullerenes.Int J Nanomedicine. 2007;2(4):639-49. Int J Nanomedicine. 2007. PMID: 18203430 Free PMC article. Review.
-
Synthesis and biological application of glyco- and peptide derivatives of fullerene C60.Eur J Med Chem. 2022 Feb 15;230:114104. doi: 10.1016/j.ejmech.2022.114104. Epub 2022 Jan 10. Eur J Med Chem. 2022. PMID: 35051749 Review.
Cited by
-
In Vivo Imaging of [60]Fullerene-Based Molecular Spherical Nucleic Acids by Positron Emission Tomography.Mol Pharm. 2023 Oct 2;20(10):5043-5051. doi: 10.1021/acs.molpharmaceut.3c00370. Epub 2023 Aug 2. Mol Pharm. 2023. PMID: 37531591 Free PMC article.
-
Synthesis of an Azide- and Tetrazine-Functionalized [60]Fullerene and Its Controlled Decoration with Biomolecules.ACS Omega. 2021 Dec 31;7(1):1329-1336. doi: 10.1021/acsomega.1c05955. eCollection 2022 Jan 11. ACS Omega. 2021. PMID: 35036794 Free PMC article.
-
Fulleropeptide esters as potential self-assembled antioxidants.Beilstein J Nanotechnol. 2015 Apr 27;6:1065-71. doi: 10.3762/bjnano.6.107. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 26171283 Free PMC article.
-
Behavioral Impairments and Oxidative Stress in the Brain, Muscle, and Gill Caused by Chronic Exposure of C70 Nanoparticles on Adult Zebrafish.Int J Mol Sci. 2019 Nov 18;20(22):5795. doi: 10.3390/ijms20225795. Int J Mol Sci. 2019. PMID: 31752171 Free PMC article.
-
Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model.Nanomaterials (Basel). 2021 Feb 18;11(2):513. doi: 10.3390/nano11020513. Nanomaterials (Basel). 2021. PMID: 33670509 Free PMC article.
References
-
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163.
-
- Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives: model building studies and experimental verification. J Am Chem Soc. 1993;115(15):6506–6509.
-
- Pantarotto D, Tagmatarchis N, Bianco A, Prato M. Synthesis and biological properties of fullerene-containing amino acids and peptides. Mini Rev Med Chem. 2004;4(7):805–814. - PubMed
-
- Foley S, Crowley C, Smaihi M, et al. Cellular localisation of a water-soluble fullerene derivative. Biochem Bioph Res Commun. 2002;294(1):116–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

