Glutamate pays its own way in astrocytes
- PMID: 24379804
- PMCID: PMC3863760
- DOI: 10.3389/fendo.2013.00191
Glutamate pays its own way in astrocytes
Abstract
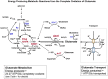
In vitro and in vivo studies have shown that glutamate can be oxidized for energy by brain astrocytes. The ability to harvest the energy from glutamate provides astrocytes with a mechanism to offset the high ATP cost of the uptake of glutamate from the synaptic cleft. This brief review focuses on oxidative metabolism of glutamate by astrocytes, the specific pathways involved in the complete oxidation of glutamate and the energy provided by each reaction.
Keywords: astrocytes; glutamate; glutamate dehydrogenase; oxidative metabolism; pyruvate recycling pathway.
Figures

Similar articles
-
Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging.Philos Trans R Soc Lond B Biol Sci. 1999 Jul 29;354(1387):1155-63. doi: 10.1098/rstb.1999.0471. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10466143 Free PMC article. Review.
-
Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis?Neurochem Int. 2013 Oct;63(4):244-58. doi: 10.1016/j.neuint.2013.06.015. Epub 2013 Jul 6. Neurochem Int. 2013. PMID: 23838211 Free PMC article. Review.
-
Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis.J Cereb Blood Flow Metab. 2007 Feb;27(2):219-49. doi: 10.1038/sj.jcbfm.9600343. Epub 2006 Jul 12. J Cereb Blood Flow Metab. 2007. PMID: 16835632 Review.
-
Glutamate oxidation in astrocytes: Roles of glutamate dehydrogenase and aminotransferases.J Neurosci Res. 2016 Dec;94(12):1561-1571. doi: 10.1002/jnr.23908. Epub 2016 Sep 15. J Neurosci Res. 2016. PMID: 27629247 Review.
-
Effects of adrenergic agents on intracellular Ca2+ homeostasis and metabolism of glucose in astrocytes with an emphasis on pyruvate carboxylation, oxidative decarboxylation and recycling: implications for glutamate neurotransmission and excitotoxicity.Neurotox Res. 2012 May;21(4):405-17. doi: 10.1007/s12640-011-9296-1. Epub 2011 Dec 23. Neurotox Res. 2012. PMID: 22194159
Cited by
-
Two Metabolic Fuels, Glucose and Lactate, Differentially Modulate Exocytotic Glutamate Release from Cultured Astrocytes.Neurochem Res. 2021 Oct;46(10):2551-2579. doi: 10.1007/s11064-021-03340-y. Epub 2021 May 31. Neurochem Res. 2021. PMID: 34057673 Free PMC article.
-
Age-Associated Upregulation of Glutamate Transporters and Glutamine Synthetase in Senescent Astrocytes In Vitro and in the Mouse and Human Hippocampus.ASN Neuro. 2023 Jan-Dec;15:17590914231157974. doi: 10.1177/17590914231157974. ASN Neuro. 2023. PMID: 36815213 Free PMC article.
-
Astrocytes and the Warning Signs of Intracerebral Hemorrhagic Stroke.Neural Plast. 2018 Feb 4;2018:7301623. doi: 10.1155/2018/7301623. eCollection 2018. Neural Plast. 2018. PMID: 29531526 Free PMC article. Review.
-
Effects of COX inhibition and LPS on formalin induced pain in the infant rat.Dev Neurobiol. 2015 Oct;75(10):1068-79. doi: 10.1002/dneu.22230. Epub 2014 Sep 13. Dev Neurobiol. 2015. PMID: 25205468 Free PMC article.
-
Glutamate and ATP at the Interface Between Signaling and Metabolism in Astroglia: Examples from Pathology.Neurochem Res. 2017 Jan;42(1):19-34. doi: 10.1007/s11064-016-1848-6. Epub 2016 Feb 25. Neurochem Res. 2017. PMID: 26915104 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

