Environmental reservoirs and mechanisms of persistence of Vibrio cholerae
- PMID: 24379807
- PMCID: PMC3863721
- DOI: 10.3389/fmicb.2013.00375
Environmental reservoirs and mechanisms of persistence of Vibrio cholerae
Abstract
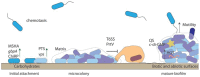
It is now well accepted that Vibrio cholerae, the causative agent of the water-borne disease cholera, is acquired from environmental sources where it persists between outbreaks of the disease. Recent advances in molecular technology have demonstrated that this bacterium can be detected in areas where it has not previously been isolated, indicating a much broader, global distribution of this bacterium outside of endemic regions. The environmental persistence of V. cholerae in the aquatic environment can be attributed to multiple intra- and interspecific strategies such as responsive gene regulation and biofilm formation on biotic and abiotic surfaces, as well as interactions with a multitude of other organisms. This review will discuss some of the mechanisms that enable the persistence of this bacterium in the environment. In particular, we will discuss how V. cholerae can survive stressors such as starvation, temperature, and salinity fluctuations as well as how the organism persists under constant predation by heterotrophic protists.
Keywords: biofilms; chitin; predation; protozoa; starvation adaptation; stress; viable but non-culturable; zooplankton.
Figures


References
-
- Abd H., Saeed A., Weintraub A., Nair G. B, Sandström G. (2007). Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii. FEMS Microbiol. Ecol. 60 33–39 10.1111/j.1574-6941.2006.00254.x - DOI - PubMed
-
- Abd H., Shanan S., Saeed A, Sandström G. (2011). Survival of Vibrio cholerae inside Acanthamoeba and detection of both microorganisms from natural water samples may point out the amoeba as a protozoal host for V. cholerae. J. Bacteriol. Parasitol. 4 109
-
- Abd H., Weintraub A, Sandström G. (2004). Interaction between Vibrio cholerae and Acanthamoeba castellanii. Microb. Ecol. Health Dis. 16 51–57 10.1080/08910600410029190 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

