Immunological function restoration with lopinavir/ritonavir versus efavirenz containing regimens in HIV-infected patients: a randomized clinical trial
- PMID: 24380397
- PMCID: PMC4010173
- DOI: 10.1089/AID.2013.0185
Immunological function restoration with lopinavir/ritonavir versus efavirenz containing regimens in HIV-infected patients: a randomized clinical trial
Abstract
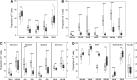
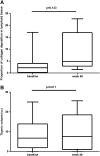
CD4(+) count increase has been reported to be different with lopinavir/r (LPV/r) and efavirenz (EFV)-containing regimens. The different effect of these two regimens on other immune function parameters and the relationship with the gain of CD4(+) count have not been assessed in a randomized clinical trial. Fifty antiretroviral treatment (cART) naïve HIV-infected individuals were randomized to receive LPV/r or EFV both with tenofovir/emtricitabine for 48 weeks. A substudy of immunological function restoration was performed in 22 patients (LPV/r n=10 and EFV n=12). Activation, thymic function, apoptosis, senescence, exhaustion, Treg cells, interleukin (IL)-7-receptor/IL-7 system, thymic volume, and lymphoid tissue fibrosis were evaluated at baseline and at week 48. Both groups experienced a CD4(+) count increase that was higher in the EFV group (ΔCD4(+) 88 vs. 315 cells/μl LPV/r vs. EFV, respectively, p<0.001). Despite this difference in CD4(+) gain, the change in other immune function parameters was similar in both treatment groups. Most of parameters evaluated tended to normalize after 48 weeks of cART. A significant decrease in levels of activation, senescence, exhaustion, and apoptosis on CD4(+) and CD8(+) T cells (p<0.001 for all) and a significant increase in markers of thymic function, IL-7 receptor, and in the levels of central memory CD4(+) T cells and naive subsets of CD8(+) T cells (p<0.001 for all) with respect to baseline values were observed without any difference between groups. These data indicate that the differences in CD4(+) gain with different cART regimens are not immunologically meaningful and might explain the similar clinical efficacy of these regimens.
Figures


References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. : Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853–860 - PubMed
-
- Thompson MA, Aberg JA, Hoy JF, et al. : Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA 2012;308(4):387–402 - PubMed
-
- Manfredi R, Calza L, and Chiodo F: First-line efavirenz versus lopinavir-ritonavir-based highly active antiretroviral therapy for naive patients. AIDS 2004;18(17):2331–2333 - PubMed
-
- Torti C, Maggiolo F, Patroni A, et al. : Exploratory analysis for the evaluation of lopinavir/ritonavir-versus efavirenz-based HAART regimens in antiretroviral-naive HIV-positive patients: Results from the Italian MASTER Cohort. J Antimicrobe Chemother 2005;56(1):190–195 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

