Hypofractionated intensity modulated radiotherapy with temozolomide in newly diagnosed glioblastoma multiforme
- PMID: 24380758
- PMCID: PMC4299456
- DOI: 10.1016/j.jocn.2013.09.005
Hypofractionated intensity modulated radiotherapy with temozolomide in newly diagnosed glioblastoma multiforme
Abstract
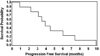
We conducted a phase I study to determine (a) the maximum tolerated dose of peri-radiation therapy temozolomide (TMZ) and (b) the safety of a selected hypofractionated intensity modulated radiation therapy (HIMRT) regimen in glioblastoma multiforme (GBM) patients. Patients with histological diagnosis of GBM, Karnofsky performance status (KPS)≥ 60 and adequate bone marrow function were eligible for the study. All patients received peri-radiation TMZ; 1 week before the beginning of radiation therapy (RT), 1 week after RT and for 3 weeks during RT. Standard 75 mg/m(2)/day dose was administered to all patients 1 week post-RT. Dose escalation was commenced at level I: 50mg/m(2)/day, level II: 65 mg/m(2)/day and level III: 75 mg/m(2)/day for 4 weeks. HIMRT was delivered at 52.5 Gy in 15 fractions to the contrast enhancing lesion (or surgical cavity) plus the surrounding edema plus a 2 cm margin. Six men and three women with a median age of 67 years (range, 44-81) and a median KPS of 80 (range, 80-90) were enrolled. Three patients were accrued at each TMZ dose level. Median follow-up was 10 months (range, 1-15). Median progression free survival was 3.9 months (95% confidence interval [CI]: 0.9-7.4; range, 0.9-9.9 months) and the overall survival 12.7 months (95% CI: 2.5-17.6; range, 2.5-20.7 months). Time spent in a KPS ≥ 70 was 8.1 months (95% CI: 2.4-15.6; range, 2.4-16 months). No instance of irreversible grade 3 or higher acute toxicity was noted. HIMRT at 52.5 Gy in 15 fractions with peri-RT TMZ at a maximum tolerated dose of 75 mg/m(2)/day for 5 weeks is well tolerated and is able to abate treatment time for these patients.
Keywords: Concurrent; Glioblastoma multiforme; Hypofractionated; Temozolomide.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Figures
References
-
- Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol. 2002;64:259–273. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Shapiro WR, Young DF. Treatment of malignant glioma. A controlled study of chemotherapy and irradiation. Arch Neurol. 1976;33:494. - PubMed
-
- Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical