Brain metabolic dysfunction at the core of Alzheimer's disease
- PMID: 24380887
- PMCID: PMC4550323
- DOI: 10.1016/j.bcp.2013.12.012
Brain metabolic dysfunction at the core of Alzheimer's disease
Abstract
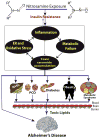
Growing evidence supports the concept that Alzheimer's disease (AD) is fundamentally a metabolic disease with molecular and biochemical features that correspond with diabetes mellitus and other peripheral insulin resistance disorders. Brain insulin/IGF resistance and its consequences can readily account for most of the structural and functional abnormalities in AD. However, disease pathogenesis is complicated by the fact that AD can occur as a separate disease process, or arise in association with systemic insulin resistance diseases, including diabetes, obesity, and non-alcoholic fatty liver disease. Whether primary or secondary in origin, brain insulin/IGF resistance initiates a cascade of neurodegeneration that is propagated by metabolic dysfunction, increased oxidative and ER stress, neuro-inflammation, impaired cell survival, and dysregulated lipid metabolism. These injurious processes compromise neuronal and glial functions, reduce neurotransmitter homeostasis, and cause toxic oligomeric pTau and (amyloid beta peptide of amyloid beta precursor protein) AβPP-Aβ fibrils and insoluble aggregates (neurofibrillary tangles and plaques) to accumulate in brain. AD progresses due to: (1) activation of a harmful positive feedback loop that progressively worsens the effects of insulin resistance; and (2) the formation of ROS- and RNS-related lipid, protein, and DNA adducts that permanently damage basic cellular and molecular functions. Epidemiologic data suggest that insulin resistance diseases, including AD, are exposure-related in etiology. Furthermore, experimental and lifestyle trend data suggest chronic low-level nitrosamine exposures are responsible. These concepts offer opportunities to discover and implement new treatments and devise preventive measures to conquer the AD and other insulin resistance disease epidemics.
Keywords: Advanced glycation end-products; Alzheimer's disease; Ceramides; Insulin resistance; Lifestyle; Metabolic syndrome; N-Nitrosodiethylamine; Nitrosamine; Nitrosamines; Non-alcoholic fatty liver disease; Obesity; Reactive nitrogen species; Streptozotocin; Type 3 diabetes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance – a mini-review. Gerontology. 2009;55:379–86. - PubMed
-
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. - PubMed
-
- Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Curr Alzheimer Res. 2009;6:213–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

