CCR5 as a natural and modulated target for inhibition of HIV
- PMID: 24381033
- PMCID: PMC3917431
- DOI: 10.3390/v6010054
CCR5 as a natural and modulated target for inhibition of HIV
Abstract
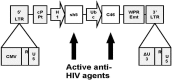
Human immunodeficiency virus type 1 (HIV-1) infection of target cells requires CD4 and a co-receptor, predominantly the chemokine receptor CCR5. CCR5-delta32 homozygosity results in a truncated protein providing natural protection against HIV infection-this without detrimental effects to the host-and transplantation of CCR5-delta32 stem cells in a patient with HIV ("Berlin patient") achieved viral eradication. As a more feasible approach gene-modification strategies are being developed to engineer cellular resistance to HIV using autologous cells. We have developed a dual therapeutic anti-HIV lentiviral vector (LVsh5/C46) that down-regulates CCR5 and inhibits HIV-1 fusion via cell surface expression of the gp41-derived peptide, C46. This construct, effective against multiple strains of both R5- and X4-tropic HIV-1, is being tested in Phase I/II trials by engineering HIV-resistant hematopoietic cells.
Figures

References
-
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/S0092-8674(00)80110-5. - DOI - PubMed
-
- Heidenhain C., Puhl G., Moench C., Lautem A., Neuhaus P. Chemokine receptor 5Delta32 mutation reduces the risk of acute rejection in liver transplantation. Ann. Transplant. 2009;14:36–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

