A 130-kDa protein 4.1B regulates cell adhesion, spreading, and migration of mouse embryo fibroblasts by influencing actin cytoskeleton organization
- PMID: 24381168
- PMCID: PMC3937661
- DOI: 10.1074/jbc.M113.516617
A 130-kDa protein 4.1B regulates cell adhesion, spreading, and migration of mouse embryo fibroblasts by influencing actin cytoskeleton organization
Abstract
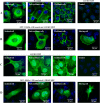
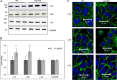
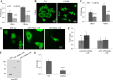
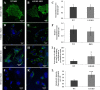
Protein 4.1B is a member of protein 4.1 family, adaptor proteins at the interface of membranes and the cytoskeleton. It is expressed in most mammalian tissues and is known to be required in formation of nervous and cardiac systems; it is also a tumor suppressor with a role in metastasis. Here, we explore functions of 4.1B using primary mouse embryonic fibroblasts (MEF) derived from wild type and 4.1B knock-out mice. MEF cells express two 4.1B isoforms: 130 and 60-kDa. 130-kDa 4.1B was absent from 4.1B knock-out MEF cells, but 60-kDa 4.1B remained, suggesting incomplete knock-out. Although the 130-kDa isoform was predominantly located at the plasma membrane, the 60-kDa isoform was enriched in nuclei. 130-kDa-deficient 4.1B MEF cells exhibited impaired cell adhesion, spreading, and migration; they also failed to form actin stress fibers. Impaired cell spreading and stress fiber formation were rescued by re-expression of the 130-kDa 4.1B but not the 60-kDa 4.1B. Our findings document novel, isoform-selective roles for 130-kDa 4.1B in adhesion, spreading, and migration of MEF cells by affecting actin organization, giving new insight into 4.1B functions in normal tissues as well as its role in cancer.
Keywords: Actin; Adhesion; Cytoskeleton; Membrane; Migration.
Figures



References
-
- Baines A. J., Lu H.-C., Bennett P. M. (2014) The Protein 4.1 family. Hub proteins in animals for organizing membrane proteins. Biochim. Biophys. Acta 1838, 605–619 - PubMed
-
- Baines A. J. (2010) The spectrin-ankyrin-4.1-adducin membrane skeleton. Adapting eukaryotic cells to the demands of animal life. Protoplasma 244, 99–131 - PubMed
-
- Peters L. L., Weier H. U., Walensky L. D., Snyder S. H., Parra M., Mohandas N., Conboy J. G. (1998) Four paralogous protein 4.1 genes map to distinct chromosomes in mouse and human. Genomics 54, 348–350 - PubMed
-
- Parra M., Gee S., Chan N., Ryaboy D., Dubchak I., Mohandas N., Gascard P. D., Conboy J. G. (2004) Differential domain evolution and complex RNA processing in a family of paralogous EPB41 (protein 4.1) genes facilitate expression of diverse tissue-specific isoforms. Genomics 84, 637–646 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

