Natural AChE Inhibitors from Plants and their Contribution to Alzheimer's Disease Therapy
- PMID: 24381530
- PMCID: PMC3744903
- DOI: 10.2174/1570159X11311040004
Natural AChE Inhibitors from Plants and their Contribution to Alzheimer's Disease Therapy
Abstract
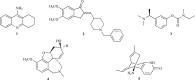
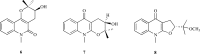
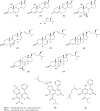
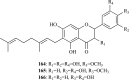
As acetylcholinesterase (AChE) inhibitors are an important therapeutic strategy in Alzheimer's disease, efforts are being made in search of new molecules with anti-AChE activity. The fact that naturally-occurring compounds from plants are considered to be a potential source of new inhibitors has led to the discovery of an important number of secondary metabolites and plant extracts with the ability of inhibiting the enzyme AChE, which, according to the cholinergic hypothesis, increases the levels of the neurotransmitter acetylcholine in the brain, thus improving cholinergic functions in patients with Alzheimer's disease and alleviating the symptoms of this neurological disorder. This review summarizes a total of 128 studies which correspond to the most relevant research work published during 2006-2012 (1st semester) on plant-derived compounds, plant extracts and essential oils found to elicit AChE inhibition.
Keywords: Alzheimer’s Disease; acetylcholinesterase inhibitors; essential oils.; plant extracts; secondary metabolites.
Figures
References
-
- Chopra K, Misra S, Kuhad A. Current perspectives on pharmacotherapy of Alzheimer’s. Expert. Opin. Pharmacother. . 2011;12:335–350. - PubMed
-
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. - PubMed
-
- Lopez S, Bastida J, Viladomat F, Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. . 2002;71: 2521–2529. - PubMed
-
- Rhee IK, van de Meent M, Ingkaninan K, Verpoorte R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combinatioin with bioactivity staining. J. Chromatogr. A . 2001;915: 217–223. - PubMed
-
- Marston A, Kissling J, Hostettmann K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. . 2002;13:51–54. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials