The effects of obesity on skeletal muscle regeneration
- PMID: 24381559
- PMCID: PMC3865699
- DOI: 10.3389/fphys.2013.00371
The effects of obesity on skeletal muscle regeneration
Abstract
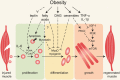
Obesity and metabolic disorders such as type 2 diabetes mellitus are accompanied by increased lipid deposition in adipose and non-adipose tissues including liver, pancreas, heart and skeletal muscle. Recent publications report impaired regenerative capacity of skeletal muscle following injury in obese mice. Although muscle regeneration has not been thoroughly studied in obese and type 2 diabetic humans and mechanisms leading to decreased muscle regeneration in obesity remain elusive, the initial findings point to the possibility that muscle satellite cell function is compromised under conditions of lipid overload. Elevated toxic lipid metabolites and increased pro-inflammatory cytokines as well as insulin and leptin resistance that occur in obese animals may contribute to decreased regenerative capacity of skeletal muscle. In addition, obesity-associated alterations in the metabolic state of skeletal muscle fibers and satellite cells may directly impair the potential for satellite cell-mediated repair. Here we discuss recent studies that expand our understanding of how obesity negatively impacts skeletal muscle maintenance and regeneration.
Keywords: leptin; lipids; lipotoxicity; muscle regeneration; obesity; satellite cells; skeletal muscle; type 2 diabetes.
Figures
References
-
- Allen D. L., Cleary A. S., Hanson A. M., Lindsay S. F., Reed J. M. (2010). CCAAT/enhancer binding protein-delta expression is increased in fast skeletal muscle by food deprivation and regulates myostatin transcription in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1592–R1601 10.1152/ajpregu.00247.2010 - DOI - PMC - PubMed
-
- Allen D. L., Cleary A. S., Speaker K. J., Lindsay S. F., Uyenishi J., Reed J. M., et al. (2008). Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am. J. Physiol. Endocrinol. Metab. 294, E918–E927 10.1152/ajpendo.00798.2007 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

