Brief monocular deprivation as an assay of short-term visual sensory plasticity in schizophrenia - "the binocular effect"
- PMID: 24381563
- PMCID: PMC3865422
- DOI: 10.3389/fpsyt.2013.00164
Brief monocular deprivation as an assay of short-term visual sensory plasticity in schizophrenia - "the binocular effect"
Abstract
Background: Visual sensory processing deficits are consistently observed in schizophrenia, with clear amplitude reduction of the visual evoked potential (VEP) during the initial 50-150 ms of processing. Similar deficits are seen in unaffected first-degree relatives and drug-naïve first-episode patients, pointing to these deficits as potential endophenotypic markers. Schizophrenia is also associated with deficits in neural plasticity, implicating dysfunction of both glutamatergic and GABAergic systems. Here, we sought to understand the intersection of these two domains, asking whether short-term plasticity during early visual processing is specifically affected in schizophrenia.
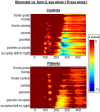
Methods: Brief periods of monocular deprivation (MD) induce relatively rapid changes in the amplitude of the early VEP - i.e., short-term plasticity. Twenty patients and 20 non-psychiatric controls participated. VEPs were recorded during binocular viewing, and were compared to the sum of VEP responses during brief monocular viewing periods (i.e., Left-eye + Right-eye viewing).
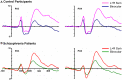
Results: Under monocular conditions, neurotypical controls exhibited an effect that patients failed to demonstrate. That is, the amplitude of the summed monocular VEPs was robustly greater than the amplitude elicited binocularly during the initial sensory processing period. In patients, this "binocular effect" was absent.
Limitations: Patients were all medicated. Ideally, this study would also include first-episode unmedicated patients.
Conclusion: These results suggest that short-term compensatory mechanisms that allow healthy individuals to generate robust VEPs in the context of MD are not effectively activated in patients with schizophrenia. This simple assay may provide a useful biomarker of short-term plasticity in the psychotic disorders and a target endophenotype for therapeutic interventions.
Keywords: EEG; biomarker; endophenotype; event-related potential; genetic liability; psychosis; vision; visual evoked potential.
Figures



References
-
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry (2007) 64:1229–4010.1001/archpsyc.64.11.1229 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

