Orexin neurons suppress narcolepsy via 2 distinct efferent pathways
- PMID: 24382351
- PMCID: PMC3904620
- DOI: 10.1172/JCI71017
Orexin neurons suppress narcolepsy via 2 distinct efferent pathways
Abstract
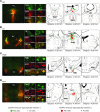
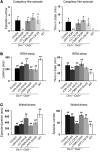
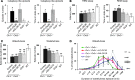
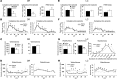
The loss of orexin neurons in humans is associated with the sleep disorder narcolepsy, which is characterized by excessive daytime sleepiness and cataplexy. Mice lacking orexin peptides, orexin neurons, or orexin receptors recapitulate human narcolepsy phenotypes, further highlighting a critical role for orexin signaling in the maintenance of wakefulness. Despite the known role of orexin neurons in narcolepsy, the precise neural mechanisms downstream of these neurons remain unknown. We found that targeted restoration of orexin receptor expression in the dorsal raphe (DR) and in the locus coeruleus (LC) of mice lacking orexin receptors inhibited cataplexy-like episodes and pathological fragmentation of wakefulness (i.e., sleepiness), respectively. The suppression of cataplexy-like episodes correlated with the number of serotonergic neurons restored with orexin receptor expression in the DR, while the consolidation of fragmented wakefulness correlated with the number of noradrenergic neurons restored in the LC. Furthermore, pharmacogenetic activation of these neurons using designer receptor exclusively activated by designer drug (DREADD) technology ameliorated narcolepsy in mice lacking orexin neurons. These results suggest that DR serotonergic and LC noradrenergic neurons play differential roles in orexin neuron-dependent regulation of sleep/wakefulness and highlight a pharmacogenetic approach for the amelioration of narcolepsy.
Figures



References
-
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

