Rationale for the sluggish oxidative addition of aryl halides to Au(I)
- PMID: 24382586
- PMCID: PMC4295554
- DOI: 10.1039/c3cc48914k
Rationale for the sluggish oxidative addition of aryl halides to Au(I)
Abstract
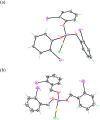
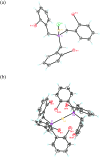
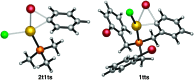
The oxidative addition of Csp(2)-Br or Csp(2)-I bonds to gold(I) does not take place even under very favorable intramolecular conditions that could form five- or six-membered gold(III) metallacycles. DFT calculations reveal that although this process could be feasible thermodynamically, it is kinetically very sluggish.
Figures
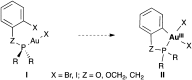
References
-
- Fürstner A., Davies P. W. Angew. Chem., Int. Ed. 2007;46:3410–3449. - PubMed
- Hashmi A. S. K. Chem. Rev. 2007;107:3180–3211. - PubMed
- Jiménez-Núñez E., Echavarren A. M. Chem. Rev. 2008;108:3326–3350. - PubMed
- Gorin D. J., Sherry B. D., Toste F. D. Chem. Rev. 2008;108:3351–3378. - PMC - PubMed
- Michelet V., Toullec P. Y., Genêt J.-P. Angew. Chem., Int. Ed. 2008;47:4268–4315. - PubMed
- Fürstner A. Chem. Soc. Rev. 2009;38:3208–3221. - PubMed
- Aubert C., Fensterbank L., Garcia P., Malacria M., Simonneau A. Chem. Rev. 2011;111:1954–1993. - PubMed
- Rudolph M., Hashmi A. S. K. Chem. Commun. 2011;47:6536–6544. - PubMed
- Obradors C., Echavarren A. M. Chem. Commun. 2015;50:16–28. - PMC - PubMed
- Obradors C., Echavarren A. M. Acc. Chem. Res. 2013 doi: 10.1021/ar400174p. - DOI - PMC - PubMed
-
- González-Arellano C., Abad A., Corma A., Garcia H., Iglesias M., Sánchez F. Angew. Chem., Int. Ed. 2007;46:1536–1538. - PubMed
- Corma A., González-Arellano C., Iglesias M., Pérez-Ferreras S., Sánchez F. Synlett. 2007:1771–1774.
- González-Arellano C., Corma A., Iglesias M., Sánchez F. Eur. J. Inorg. Chem. 2008:1107–1115.
-
- Li P., Wang L., Wang M., You F. Eur. J. Org. Chem. 2008:5946–5951.
-
- de Souza O. M. A., Bittar M. S., Mendes L. V. P., Michele C., da Silva F. Synlett. 2008:1777–1780.
-
- Yao P. J. Chem. Res. 2013;37:174–176.
LinkOut - more resources
Full Text Sources
Other Literature Sources

