The role of molecular dipole orientation in single-molecule fluorescence microscopy and implications for super-resolution imaging
- PMID: 24382708
- PMCID: PMC3992256
- DOI: 10.1002/cphc.201300880
The role of molecular dipole orientation in single-molecule fluorescence microscopy and implications for super-resolution imaging
Abstract
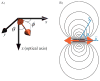
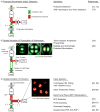
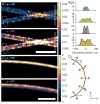
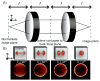
Numerous methods for determining the orientation of single-molecule transition dipole moments from microscopic images of the molecular fluorescence have been developed in recent years. At the same time, techniques that rely on nanometer-level accuracy in the determination of molecular position, such as single-molecule super-resolution imaging, have proven immensely successful in their ability to access unprecedented levels of detail and resolution previously hidden by the optical diffraction limit. However, the level of accuracy in the determination of position is threatened by insufficient treatment of molecular orientation. Here we review a number of methods for measuring molecular orientation using fluorescence microscopy, focusing on approaches that are most compatible with position estimation and single-molecule super-resolution imaging. We highlight recent methods based on quadrated pupil imaging and on double-helix point spread function microscopy and apply them to the study of fluorophore mobility on immunolabeled microtubules.
Keywords: fluorescence microscopy; molecular orientation; rotational mobility; single-molecule studies; super-resolution imaging.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Moerner WE, Fromm DP. Rev Sci Instrum. 2003;74:3597–3619.
-
- Moerner WE, Orrit M. Science. 1999;283:1670–1676. - PubMed
-
- Moerner WE. In: Single-Molecule Optical Spectroscopy and Imaging: From Early Steps to Recent Advances in Single Molecule Spectroscopy in Chemistry, Physics and Biology: Nobel Symposium 138 Proceedings. Graslund A, Rigler R, Widengren J, editors. Springer; Berlin: 2009. pp. 25–60.
-
- Betzig E, Chichester RJ. Science. 1993;262:1422–1425. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

