NAD(P)H-independent asymmetric C=C bond reduction catalyzed by ene reductases by using artificial co-substrates as the hydrogen donor
- PMID: 24382795
- PMCID: PMC4413776
- DOI: 10.1002/chem.201303897
NAD(P)H-independent asymmetric C=C bond reduction catalyzed by ene reductases by using artificial co-substrates as the hydrogen donor
Abstract
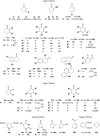
To develop a nicotinamide-independent single flavoenzyme system for the asymmetric bioreduction of C=C bonds, four types of hydrogen donor, encompassing more than 50 candidates, were investigated. Six highly potent, cheap, and commercially available co-substrates were identified that (under the optimized conditions) resulted in conversions and enantioselectivities comparable with, or even superior to, those obtained with traditional two-enzyme nicotinamide adenine dinucleotide phosphate (NAD(P)H)-recycling systems.
Keywords: alkene reduction; artificial biocatalysis; ene reductases; enzyme catalysis; hydrogen donors.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures



References
-
- Stuermer R, Hauer B, Hall M, Faber K. Curr. Opin. Chem. Biol. 2007;11:203–213. - PubMed
-
- Toogood HS, Gardiner JM, Scrutton NS. ChemCatChem. 2010;2:892–914.
-
- Oberdorfer G, Gruber K, Faber K, Hall M. Synlett. 2012;23:1857–1864.
-
- Faber K. Biotransformations in Organic Chemistry. 6. Heidelberg: Springer; 2011. pp. 140–145. th ed.,, pp. .
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

