Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function
- PMID: 24382870
- PMCID: PMC4073945
- DOI: 10.1152/physiol.00012.2013
Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function
Abstract
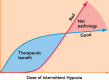
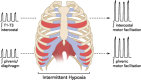
Intermittent hypoxia (IH) is most often thought of for its role in morbidity associated with sleep-disordered breathing, including central nervous system pathology. However, recent evidence suggests that the nervous system fights back in an attempt to minimize pathology by increasing the expression of growth/trophic factors that confer neuroprotection and neuroplasticity. For example, even modest ("low dose") IH elicits respiratory motor plasticity, increasing the strength of respiratory contractions and breathing. These low IH doses upregulate hypoxia-sensitive growth/trophic factors within respiratory motoneurons but do not elicit detectable pathologies such as hippocampal cell death, neuroinflammation, or systemic hypertension. Recent advances have been made toward understanding cellular mechanisms giving rise to IH-induced respiratory plasticity, and attempts have been made to harness the benefits of low-dose IH to treat respiratory insufficiency after cervical spinal injury. Our recent realization that IH also upregulates growth/trophic factors in nonrespiratory motoneurons and improves limb (or leg) function after incomplete chronic spinal injuries suggests that IH-induced plasticity is a general feature of motor systems. Collectively, available evidence suggests that low-dose IH may represent a safe and effective treatment to restore lost motor function in diverse clinical disorders that impair motor function.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures



References
-
- Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Mueller M, Hassouna I, Hannke K, Sperling S, Radyushkin K, El-Kordi A, Schulze L, Ronnenberg A, Wolf F, Brose N, Rhee JS, Zhang W, Ehrenreich H. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol 6: 37, 2008 - PMC - PubMed
-
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkN-Shc site signals neuronal surivival and local axon growth via MEK and PI3-Kinase. Neuron 27: 265–277, 2000 - PubMed
-
- Bach KB, Mitchell GS. Hypoxia-induced facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

