Respiratory rhythm generation in vivo
- PMID: 24382872
- PMCID: PMC3929116
- DOI: 10.1152/physiol.00035.2013
Respiratory rhythm generation in vivo
Abstract
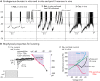
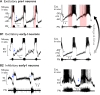
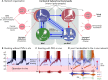
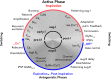
The cellular and circuit mechanisms generating the rhythm of breathing in mammals have been under intense investigation for decades. Here, we try to integrate the key discoveries into an updated description of the basic neural processes generating respiratory rhythm under in vivo conditions.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures



References
-
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143: 105–114, 2004 - PubMed
-
- Backman SB, Anders C, Ballantyne D, Röhrig N, Camerer H, Mifflin S, Jordan D, Dickhaus H, Spyer KM, Richter DW. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflügers Arch 402: 129–136, 1984 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

