Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition
- PMID: 24383403
- PMCID: PMC3880410
- DOI: 10.1186/1471-2407-14-1
Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition
Abstract
Background: Increased collagen deposition provides physical and biochemical signals to support tumor growth and invasion during breast cancer development. Therefore, inhibition of collagen synthesis and deposition has been considered a strategy to suppress breast cancer progression. Collagen prolyl-4-hydroxylase α subunit 2 (P4HA2), an enzyme hydroxylating proline residues in -X-Pro-Gly- sequences, is a potential therapeutic target for the disorders associated with increased collagen deposition. However, expression and function of P4HA2 in breast cancer progression are not well investigated.
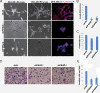
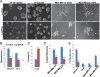
Methods: Gene co-expression analysis was performed in the published microarray datasets to identify potential regulators of collagen I, III, and IV in human breast cancer tissue. Expression of P4HA2 was silenced by shRNAs, and its activity was inhibited by 1, 4-DPCA, a prolyl-4-hydroxylase inhibitor. Three-dimensional culture assay was used to analyze roles of P4HA2 in regulating malignant phenotypes of breast cancer cells. Reduced deposition of collagen I and IV was detected by Western blotting and immunofluorescence. Control and P4HA2-silenced breast cancer cells were injected into fat pad and tail vein of SCID mice to examine effect of P4HA2 on tumor growth and lung metastasis.
Results: Using gene co-expression analysis, we showed that P4HA2 was associated with expression of Col1A1, Col3A1, and Col4A1 during breast cancer development and progression. P4HA2 mRNA levels were significantly upregulated in breast cancer compared to normal mammary tissue. Increased mRNA levels of P4HA2 correlated with poor clinical outcome in breast cancer patients, which is independent of estrogen receptor status. Silencing P4HA2 expression or treatment with the P4HA inhibitor significantly inhibited cell proliferation and suppressed aggressive phenotypes of breast cancer cells in 3D culture, accompanied by reduced deposition of collagen I and IV. We also found that knockdown of P4HA2 inhibited mammary tumor growth and metastasis to lungs in xenograft models.
Conclusion: These results suggest the critical role of P4HA2 in breast cancer progression and identify P4HA2 as a potential therapeutic target and biomarker for breast cancer progression.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

